successful completion of the questions at the end of this paper has been approved for continuing education by the bddt-n; 1.0 credit nutritional medicine and by the cnpbc; one ce hour.
1Bachelor of Health Sciences (Honours), McMaster University. Naturopathic Doctor, Canadian College of Naturopathic Medicine
2Bachelor of Health Sciences (Honours) Candidate, McMaster University.
Breaking it down
The role of acid-base balance in the pathogenesis and treatment of osteoporosis
ABSTRACT
Osteoporosis (OP) is a skeletal disorder characterized by low bone mineral density (BMD), which predisposes individuals to increased risk of skeletal fractures (Foundation 2010). In 2009, more than 22% of Canadians over the age of 50 were diagnosed with OP (Garriguet 2011). In 2010, OP accounted for $2.3 billion in Canada’s healthcare expenditures of which over 50% were utilized for acute care of managing OP-related fractures (Tarride 2012). The etiology of OP is defined by elevated osteoclastic activity relative to osteoblastic activity, which induces bone resorption and deterioration of bone tissue (Duque 2008). OP is associated with multiple risk factors including advanced age, hormone imbalance, sedentary lifestyle, and hypocalcaemia (Tucker 2001). However, Kanis et al. (Kanis 2007) found that measuring BMD in addition to clinical risk factors provides the best fracture risk prediction.
The standard first-line therapy for OP includes oral bisphosphonates combined with vitamin D and calcium (Ca) supplementation therapy, which are recommended from the age of 50 (Papaioannou 2010). However, bisphosphonates diminish in efficacy following three to five years of treatment and may cause severe adverse effects such as osteonecrosis of the jaw (Nase 2006, Rachner 2011). As such, investigation into alternative therapies is warranted. Furthermore, the role of Ca deficiency in the pathogenesis of OP fails to account for higher rates of hip fracture in Western countries with higher Ca intake relative to developing countries with lower Ca intake (FAO 2004, Poliquin 2009). As a result, alternative factors for disease progression, including those that influence the efficacy of Ca supplementation, have been investigated (FAO 2004).
It has been suggested that a diet consisting of protein and grains is associated with an increased net acid load, which is buffered via bone dissolution (Fenton 2009a, Wachman 1968). The result is a decrease in BMD and a release of calcium from bone, which is subsequently excreted through urine (Fenton 2009a). It is posited that an alkaline diet consisting of a high intake of fruits and vegetables, which are metabolized to bicarbonate (HCO3), would buffer acidity and thereby preserve Ca levels and BMD (Fenton 2009a). The objective of this article is to explore the role of acid-base balance in OP pathogenesis, as well as its potential to drive alternative therapies. The validity of this hypothesis will be explored within the context of acid-base balance in OP, dietary effects of metabolic acidosis (specifically protein and minerals such as potassium (K) and sodium (Na)), and the role of physical activity on pH homeostasis.
ACID-BASE BALANCE IN OSTEOPOROSIS
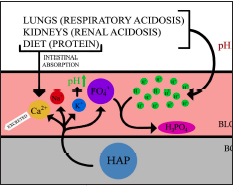
Bone dissolution is a mechanistic phosphate-buffering response to low systemic pH as a result of increased dietary acid load and respiratory and/or renal acidosis (Fig. 1) (Bushinsky 2010). It was first suggested by Wachman and Bernstein (1968) that induced chronic metabolic acidosis and prolonged utilization of the phosphate-buffer system reduces skeletal buffering capacity over time, theoretically explaining age-associated osteoporotic risk. A Western diet high in grain and animal protein has been associated with increased calciuria and net urinary acid production (Jehle 2006, Maurer 2003). This is thought to represent elevated systemic acidity and would contribute towards OP pathogenesis (Jehle 2006).
Figure 1. highlights the outcome of inadequate acid-base buffering in lungs and kidneys contributing to respiratory and renal acidosis, respectively. Although protein increases intestinal absorption of calcium, sulfur-containing amino acids in protein can also contribute towards acidosis. This systemic acid-base imbalance is in part alleviated by the skeletal system. Hydroxyapatite (HAP) typically stored in bone dissociates in response to low pH, releasing Ca, K, magnesium, and phosphate into the circulatory system. The release of phosphate ions neutralizes blood pH by forming H3PO4. Released K ions further alkalinizes systemic pH.
This effect was further substantiated through NH4Cl loading in human subjects (n=14) where bone resorptive Ca loss, demonstrated by acidosis-induced calciuria, occurred in the absence of increased intestinal Ca absorption (Lemann 2003). Furthermore, acidosis has been associated with increased osteoclastic activity in human peripheral blood in vitro and inhibition of osteoblastic bone formation in rats in vitro (Arnett 2007, Brandao-Burch 2005). In a randomized prospective trial of 161 postmenopausal women diagnosed with osteopenia, alkali treatment of 30 mEq/d oral potassium citrate was associated with 1.87%, 1.39%, and 1.98% increased BMD over one year in lumbar spine, femoral neck, and total hip, respectively (Jehle 2006).
PROTEIN
Although the link between metabolic acidosis and bone resorption has been previously established, there is significant debate over which acidogenic influences contribute to OP, as well as the extent of their impacts on systemic pH. Accumulation of acid load has been widely attributed to excessive protein intake and acid buildup via elevated metabolism of sulfur-containing amino acids (methionine and cysteine) found in higher quantities in animal protein (Fenton 2009a, Poupin 2012, Remer 2000). However, the basis of correlations between protein and systemic acidosis relies on the assumption that urinary Ca excretion induced by protein intake is representative of skeletal Ca loss. Instead, a report evaluating acid balance assessment techniques asserted that the quantification of acid balance requires direct evaluation of intestinal absorption and cannot be indirectly assessed through urinary composition (Lemann 2003). Recent theory proposes that the protein-induced calciuric effect may instead be a byproduct of increased intestinal Ca absorption (Bonjour 2009, Levis 2012). It is thought that dietary protein stimulates gastric acid production and increases Ca solubility and bioavailability, thus contributing towards urinary Ca excretion (Kerstetter 2005). Protein causes increased urinary Ca loss, although the source of the excreted Ca is uncertain. These confounding factors have not been recognized until recently.
Population-based, protective effects of protein intake on BMD have been previously observed (Tucker 2001). In the Framingham Osteoporosis Study, dietary habits and BMD measurements of 615 elderly participants were analyzed in a longitudinal cohort study (Tucker 2001). (valium) Animal protein was associated with a stimulating or protective effect on bone mass, and participants with the greatest protein intake had the highest BMD (Tucker 2001). The highest quartile of protein intake consistently showed the lowest BMD loss over four years at the femoral neck and lumbar spine (Tucker 2001). Although study participants were mainly elderly, and one third of subjects had protein intake below the recommended daily allowance; protein was shown to be important in stimulating and maintaining bone growth in older adults (Tucker 2001). Although the mechanism of that effect remained identified, Gaffney-Stomberg et al. (2009) characterized the beneficial effects of protein intake on bone health through mechanisms involving insulin-like growth factor-1 (IGF-1) and parathyroid hormone (PTH) – factors associated with bone growth and resorption, respectively.
In a randomized controlled feeding study of 27 postmenopausal women, high protein intake (20% of daily energy intake) in low-Ca intake individuals (~675 mg/d) significantly increased Ca isotope absorption (29.5% vs. 26.0%) and compensated for the majority of a 0.5 mmol/d increase in calciuria (Hunt 2009). Additionally, in a randomized crossover study comparing (1) low protein and low-potential renal acid load (LPLP) to (2) high protein and high-potential renal acid load (HPHP) diets in 16 postmenopausal women, HPHP diet was associated with increased intestinal Ca absorption (26.5% vs 22.3%, p<0.05) (Cao 2011). Although neither dietary intervention (LPLP and HPHP) was found to influence markers of bone metabolism, upregulation of serum IGF-1, and downregulation of PTH substantiated proposed benefits of protein on bone even after accounting for renal acid load (Cao 2011).
In the context of acid-base balance, it is important to recognize that the compensatory effect of increased intestinal Ca absorption does not completely counterbalance calciuric outcomes (Cao 2011, Hunt 2009). The minimal discrepancy in Ca absorption and excretion may yet contribute towards the etiology of OP through acidosis over time (New 2003). Nonetheless, substantial evidence demonstrating the beneficial effects of protein on bone health, especially in elderly and individuals with low Ca intake, cannot be ignored cannot be ignored. Furthermore, it has been suggested that the current recommended daily intake of 0.8g/kg prevents protein deficiency but is insufficient for optimization of bone health, especially in the elderly (Bushinsky 2010, Gaffney-Stomberg 2009).
POTASSIUM
Aside from high protein intake, the modern Western diet is characterized by energy-dense nutrient-poor foods, including fats, sugars, and Na (Frassetto 2008). It also involves decreased intake of K and HCO3-precursor-rich plants, since the most common plant food ingested is cultivated cereal grain, which yields a net acid load (Sebastian 2002). This diet was only adopted in the last 10 000 years, which is too recent for evolutionary mechanisms to have adjusted in terms of core metabolic machinery (Eaton 1988, Tobian 1988).
Prior to the modern diet, humans ingested a higher ratio of K-to-Na and so the kidneys are programmed to excrete more K than Na (Frassetto 2008). However, this evolutionary mechanism has persisted despite the inversion of the dietary K-to-Na ratio (Eaton 1996). It has been demonstrated that interstitial fluid of bone contains higher concentrations of K and Na than Ca and phosphorus (Krieger 2004). K content is directly related to the amount of consumed and absorbed K and is used by the skeletal system to buffer metabolic acid load (Green 1991, Krieger 2004). Thus, it is important to maintain high levels of K, regardless of food source (Rafferty 2005).
Fruits and vegetables may be dually helpful as they contain both K and HCO3 precursors (Frassetto 2005). It has been demonstrated that in postmenopausal women, higher intake of fruit were positively associated with increased BMD (Chen 2001). Furthermore, increased K content of plant protein relative to animal protein resulted in lowered risk for chronic metabolic acidosis (Deriemaeker 2010).
SODIUM
It has been postulated that high sodium chloride (NaCl) content and low proportion of plant foods in the Western diet induces metabolic acidosis (Frassetto 2008). This effect is compounded by age-related decrease in renal function (Frassetto 2008). It has been suggested that 50-100% of the diet’s net acid load can be attributed to high NaCl intake (Frassetto 2007). Therefore, decreasing the amount of NaCl intake or buffering NaCl with dietary base precursors could help maintain bone mass (Buehlmeier 2012). In a randomized crossover trial, eight male volunteers were randomized into two study campaigns: (1) the experimental group, receiving 90 mmol/d of potassium bicarbonate (KHCO3) to counteract a high NaCl diet (7.3 mmol/kg/d compared to the average NaCl diet of 2.6 mmol/kg/d) and (2) the control group, receiving only a NaCl-rich diet (7.3 mmol/kg/d) (Buehlmeier 2012). It was found that KHCO3 supplementation for ten days decreased NaCl-induced calciuria by 12% (p<0.05), indicating that intake of dietary base precursors may have a protective effect against bone mass loss (Buehlmeier 2012). The authors propose that decreased calciuria is attributed to increased Ca deposition in bone, though these results could be an indication of increased Ca utilization in other body systems(Buehlmeier 2012). Additionally, the adverse effects of NaCl were more persistent than the protective effects of KHCO3 (Buehlmeier 2012). The transient KHCO3 effect is thought to be due to relatively low amounts of KHCO3 (i.e. 90 mmol/d), suggesting that increased levels of KHCO3 may cause a stronger and more protective effect (Buehlmeier 2012).

Considerations of
Data Presented
It has been previously shown that both dietary Ca and urinary Na excretion were significantly correlated with changes in bone mass over a two-year period at the hip and ankle (Devine 1995). Furthermore, the reduction of Na intake has been reported to have beneficial skeletal effects on participants undergoing the Dietary Approaches to Stop Hypertension diet for a month (Lin 2003). In addition, higher dietary Na in young men and women led to increased 1,25-dihydroxyvitamin D and increased intestinal Ca absorption which accounted for Na-induced calciuria (Breslau 1982). It is interesting to note, however, that postmenopausal women did not demonstrate these increased 1,25-dihydroxyvitamin D levels, which may suggest that this population is unable to compensate for the calciuria caused by increased Na ingestion (Breslau 1985). Finally, Frassetto et al. (2008) conclusively demonstrated that dietary NaCl drives urinary Ca excretion, increases bone resorption, and increases the relative rates of bone resorption to bone formation
PHYSICAL ACTIVITY
A lack of research surrounds the effects of physical activity on the body’s acid-base balance. Most research focuses on physical activity as a potential confounder instead of its role in maintaining acid-base homeostasis. Research on physical activity mainly centers on preventative measures rather than therapeutic options for OP (Borer 2005, Guadalupe-Grau 2009). Preventative treatment options include high-resistance physical activity to increase the peak BMD levels in youths, and yoga and tai chi to improve flexibility and reduce fractures among elderly persons (Borer 2005, Guadalupe-Grau 2009). There has been some discussion on the idea that deep breathing associated with yoga and other physical activities could be involved with acid-base balance within the body, as the body regulates acid-base homeostasis through the exhalation of CO2 through the lungs (Horowitz 2009). However, there has not been rigorous research done on this topic, and in order to determine the effects of physical activity on the body’s acid-base homeostasis, further studies need to be conducted.
CONCLUSION
The physiochemical basis for acid-base balance in OP is founded upon sound principles but is not consistently supported by past studies, some of which used inadequate measures of systemic pH (Fenton 2009b, Lemann 2003, Poupin 2012, Remer 2000). Recent studies have found beneficial effects of protein intake on BMD and calcium absorption (Cao 2011, Tucker 2001). Furthermore, HCO3 and K derived from fruit and vegetable metabolism may be promising with regard to net acid load reduction and OP prevention, in addition to other beneficial effects on overall health (Frassetto 2005). However, as contended previously, the Western diet may not be solely responsible for OP pathogenesis since other countries with different dietary patterns exhibit similar patterns in OP prevalence (Fenton 2011). Promising alternative therapies that may aid in the prevention of OP include moderate aerobic exercises, weight-bearing activities, and balance exercises (Borer 2005, Fishman 2009, Guadalupe-Grau 2009, Maciaszek 2007). The clinical implications of findings reviewed in this paper are summarized below (Table 1). Additional etiologic factors may include sunlight exposure, physical labour, and genetic predisposition in these populations (Fenton 2011). Nonetheless, more primary research is needed to fully understand the role of acid-base balance in OP pathogenesis as well as its treatment potential
REFERENCES
Arnett TR, editor. Acid–base regulation of bone metabolism. International Congress Series; 2007: Elsevier.
Bonjour J-P, Chevalley T. The dietary protein-acidosis hypothesis in the pathophysiology of osteoporosis. IBMS BoneKEy. 2009;6(7):254-8.
Borer KT. Physical activity in the prevention and amelioration of osteoporosis in women.Sports Medicine. 2005;35(9):779-830.
Brandao-Burch A, Utting J, Orriss I, Arnett T. Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcified tissue international. 2005;77(3):167-74.
Breslau N, Sakhaee K, Pak C. Impaired adaptation to salt-induced urinary calcium losses in postmenopausal osteoporosis. Transactions of the Association of American Physicians. 1985;98:107.
Breslau NA, McGuire JL, Zerwekh JE, Pak CY.The role of dietary sodium on renal excretion and intestinal absorption of calcium and on vitamin D metabolism.Journal of Clinical Endocrinology & Metabolism. 1982;55(2):369-73.
Buehlmeier J, Frings-Meuthen P, Remer T, Maser-Gluth C, Stehle P, Biolo G, et al. Alkaline Salts to Counteract Bone Resorption and Protein Wasting Induced by High Salt Intake: Results of a Randomized Controlled Trial. Journal of Clinical Endocrinology & Metabolism. 2012;97(12):4789-97.
Bushinsky DA. Acidosis and bone. Nutritional Influences on Bone Health: Springer; 2010. p. 161-6.
Cao JJ, Johnson LK, Hunt JR. A diet high in meat protein and potential renal acid load increases fractional calcium absorption and urinary calcium excretion without affecting markers of bone resorption or formation in postmenopausal women. The Journal of nutrition. 2011;141(3):391-7.
Chen Y, Ho S, Lee R, Lam S, Woo J. Fruit intake is associated with better bone mass among Hong Kong Chinese early postmenopausal women. J Bone Miner Res. 2001;16(suppl 1):S386.
Deriemaeker P, Aerenhouts D, Hebbelinck M, Clarys P. Nutrient Based Estimation of Acid-Base Balance in Vegetarians and Non-vegetarians. Plant foods for human nutrition. 2010;65(1):77-82.
Devine A, Criddle RA, Dick IM, Kerr DA, Prince RL. A longitudinal study of the effect of sodium and calcium intakes on regional bone density in postmenopausal women.The American journal of clinical nutrition. 1995;62(4):740-5.
Duque G, Kiel DP. Osteoporosis in Older Persons: Pathophysiology and Therapeutic Approach: Springer; 2008.
Eaton SB, Eaton SB, Konner MJ, Shostak M. An evolutionary perspective enhances understanding of human nutritional requirements. The Journal of nutrition. 1996;126(6):1732-40.
Eaton SB, Konner M, Shostak M. Stone agers in the fast lane: chronic degenerative diseases in evolutionary perspective. The American journal of medicine. 1988;84(4):739-49.
FAO W. Vitamin and mineral requirements in human nutrition, Geneva.World Health Organization. 2004.
Fenton TR, Lyon AW. Milk and acid-base balance: proposed hypothesis versus scientific evidence. Journal of the American College of Nutrition. 2011;30(5 Supplement 1):471S-5S.
Fenton TR, Lyon AW, Eliasziw M, Tough SC, Hanley DA.Meta‐Analysis of the Effect of the Acid‐Ash Hypothesis of Osteoporosis on Calcium Balance.Journal of Bone and Mineral Research. 2009;24(11):1835-40.
Fenton TR, Lyon AW, Eliasziw M, Tough SC, Hanley DA. Phosphate decreases urine calcium and increases calcium balance: a meta-analysis of the osteoporosis acid-ash diet hypothesis. Nutr J. 2009;8:41.
Fishman LM. Yoga for osteoporosis: A pilot study. Topics in Geriatric Rehabilitation. 2009;25(3):244-50.
Foundation NO.Clinician’s guide to prevention and treatment of osteoporosis.National Osteoporosis Foundation Washington DC; 2010.
Frassetto L, Morris RC, Sebastian A. Long-term persistence of the urine calcium-lowering effect of potassium bicarbonate in postmenopausal women. Journal of Clinical Endocrinology & Metabolism. 2005;90(2):831-4.
Frassetto LA, Morris RC, Sebastian A. Dietary sodium chloride intake independently predicts the degree of hyperchloremic metabolic acidosis in healthy humans consuming a net acid-producing diet. American Journal of Physiology-Renal Physiology. 2007;293(2):F521-F5.
Frassetto LA, Morris RC, Sellmeyer DE, Sebastian A. Adverse effects of sodium chloride on bone in the aging human population resulting from habitual consumption of typical American diets. The Journal of nutrition. 2008;138(2):419S-22S.
Gaffney-Stomberg E, Insogna KL, Rodriguez NR, Kerstetter JE. Increasing dietary protein requirements in elderly people for optical muscle and bone health. Journal of the American Geriatrics Society. 2009;57:1073-9.
Garriguet D. Bone health: Osteoporosis, calcium and vitamin D. In: Canada S, editor. Health Reports 2011.
Guadalupe-Grau A, Fuentes T, Guerra B, Calbet JA. Exercise and bone mass in adults. Sports Medicine. 2009;39(6):439-68.
Horowitz S. Acid–Base Balance, Health, and Diet.Alternative and Complimentary Therapies. 2009;15(6):292-7.
Health Canada. (June 8, 2012). Sodium in Canada. In Health Canada – Food and Nutrition. Retrieved April 1, 2013, from http://www.hc-sc.gc.ca/fn-an/nutrition/sodium/index-eng.php.
Hunt JR, Johnson LK, Roughead ZF. Dietary protein and calcium interact to influence calcium retention: a controlled feeding study. The American journal of clinical nutrition. 2009;89(5):1357- 65.
Jehle S, Zanetti A, Muser J, Hulter HN, Krapf R. Partial neutralization of the acidogenic Western diet with potassium citrate increases bone mass in postmenopausal women with osteopenia.Journal of the American Society of Nephrology. 2006;17(11):3213-22.
Kanis J, Odén A, Johnell O, Johansson H, De Laet C, Brown J, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporosis International. 2007;18(8):1033-46.
Kerstetter JE, O’Brien KO, Caseria DM, Wall DE, Insogna KL.The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women.Journal of Clinical Endocrinology & Metabolism. 2005;90(1):26-31.
Krieger NS, Frick KK, Bushinsky DA.Mechanism of acid-induced bone resorption.Current opinion in nephrology and hypertension. 2004;13(4):423-36.
Lemann J, Bushinsky DA, Hamm LL. Bone buffering of acid and base in humans.American Journal of Physiology-Renal Physiology. 2003;285(5):F811-F32.
Levis S, Lagari VS. The Role of Diet in Osteoporosis Prevention and Management.Current osteoporosis reports. 2012;10(4):296- 302.
Lin P-H, Ginty F, Appel LJ, Aickin M, Bohannon A, Garnero P, et al. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. The Journal of nutrition. 2003;133(10):3130-6.
Maciaszek J, Osiński W, Szeklicki R, Stemplewski R. Effect of Tai Chi on body balance: randomized controlled trial in men with osteopenia or osteoporosis. The American journal of Chinese medicine. 2007;35(01):1-9.
Maurer M, Riesen W, Muser J, Hulter HN, Krapf R. Neutralization of Western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. American Journal of Physiology-Renal Physiology. 2003;284(1):F32-F40.
Nase JB, Suzuki JB. Osteonecrosis of the jaw and oral bisphosphonate treatment. J Am Dent Assoc. 2006;137(8):1115- 9.
New SA. Intake of fruit and vegetables: Implications for bone health. Proceedings of the Nutrition Society. 2003; 52:889-99.
Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. Canadian Medical Association Journal. 2010;182(17):1864-73.
Poliquin S, Joseph L, Gray-Donald K. Calcium and vitamin D intakes in an adult Canadian population.Canadian Journal of Dietetic Practice and Research. 2009;70(1):21-7.
Poupin N, Calvez J, Lassale C, Chesneau C, Tomé D. Impact of the diet on net endogenous acid production and acid–base balance. Clinical Nutrition. 2012;31(3):313-21.
Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. The Lancet. 2011;377(9773):1276-87.
Rafferty K, Davies KM, Heaney RP. Potassium intake and the calcium economy.Journal of the American College of Nutrition. 2005;24(2):99-106.
Remer T, editor. ACID‐BASE IN RENAL FAILURE: Influence of Diet on Acid‐Base Balance. Seminars in Dialysis; 2000: Wiley Online Library.
Sebastian A, Frassetto LA, Sellmeyer DE, Merriam RL, Morris RC. Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors.The American journal of clinical nutrition. 2002;76(6):1308-16.
Tarride J-E, Hopkins R, Leslie W, Morin S, Adachi J, Papaioannou A, et al. The burden of illness of osteoporosis in Canada.Osteoporosis International. 2012;23(11):2591-600.
Tobian L.The Volhard lecture.Potassium and sodium in hypertension. Journal of hypertension Supplement: official journal of the International Society of Hypertension. 1988;6(4):S12.
Tucker KL, Hannan MT, Kiel DP. The acid-base hypothesis: diet and bone in the Framingham Osteoporosis Study. European journal of nutrition. 2001;40(5):231-7.
Wachman A, Bernstein DS. Diet and osteoporosis.The Lancet. 1968;291(7549):958-9.