successful completion of the questions at the end of this paper has been approved for continuing education by the bddt-n; 1.0 credit parenteral therapy and by the cnpbc; one ce hour.
Effectiveness of Intravenous Vitamin C in Combination with Conventional Chemotherapy in Cancer Treatment
A Review
James Bao1, Chloe Haldane1, Yvonne Lee2, Brian Li1, Olesya Petrenko1, Christopher Wang1, Wendy Zhou2, Jordan Robertson ND3
¹ Bachelor of Health Sciences (Honours), McMaster University
² Bachelor of Health Sciences (Honours) Candidate, McMaster University
3 Bachelor of Health Sciences, McMaster University, Naturopathic Doctor, Canadian College of Naturopathic Medicine.
Many integrative practitioners are choosing to supplement conventional chemotherapy with intravenous vitamin C (IVC) in cancer patients. Currently, preliminary research has demonstrated the potential benefits of IVC on the length and quality of life in cancer patients. Human- level evidence at this time is limited, yet suggests a role for IV-C as an adjunct treatment for many cancers. There is a strong need for additional research to establish situations the therapy is best suited for, further develop the evidence base of safety in conjunction with conventional therapies, and more clearly delineate the specific outcomes expected from the therapy.
Introduction
The potential benefits of intravenous vitamin C (IVC) in the treatment of cancer have been debated for several decades. The landmark study conducted by Cameron and Pauling in 1976 was the first to highlight the potential use of vitamin C as a form of medication in the treatment of advanced cancer patients. Since then, clinical studies have reaffirmed that cancer patients exhibit abnormally low plasma ascorbic acid (AA) levels secondary to the disease or treatment, and it is suggested that this contributes to a decrease in survival rate (Shah 2009). Currently, IVC research has been extended to include in vitro, in vivo, and human clinical trials on several cancer types.
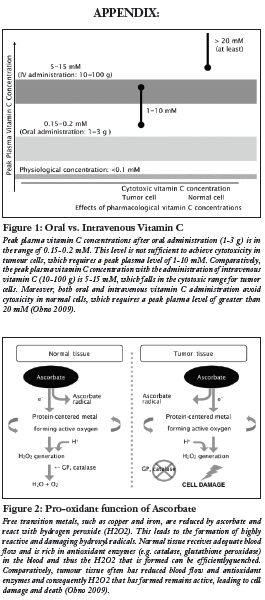
Preclinical evidence has provided a number of antitumourigenic mechanisms of high dose vitamin C in cancer (Mikirova 2008, Ohno 2009). Since active transporters tightly regulate intestinal absorption of vitamin C, a desirable concentration of AA can only be achieved via intravenous injection, as opposed to oral administration (Padayatty 2004) (see Figure 1). Although results from pre-clinical studies in mice have shown a decrease in tumour growth, human clinical trials, thus far, have not found similar results (Chen 2008, Hoffer 2008, Monti 2012). Rather, these studies show that IVC has limited adverse effects and is a safe and viable treatment to increase quality of life in advanced cancer patients by decreasing the side effects from chemotherapy. Mechanistic studies utilizing cell lines and animal models demonstrate promising results in terms of changing the disease progression itself (Chen 2008). This review hopes to examine any progressions on this theory through evidence provided by recent human clinical trials.
Mechanisms of action based on cell culture studies
Before vitamin C can be investigated as a possible novel cancer treatment, it is essential first to learn whether ascorbate can act as an anticancer agent in cell culture, and if so, by what mechanisms (Chen 2005). Since the 1980s, the involvement of ascorbic acid has been referenced in a number of studies examining cancer cell proliferation specifically in melanoma cells (Bram 1980), oral squamous cell carcinoma and salivary gland tumour cells (Sakagami 2000). In 2005, Chen and colleagues evaluated the use of AA on human cancer cell lines (lymphoma and three types of breast cancer cell types) and mouse cancer cells (lung, kidney, colon and melanoma) through one-hour incubations at pharmacologic concentrations of 0.3-20 mM of vitamin C (Chen 2005).The researchers observed that AA supplementation inhibited growth of breast cancer cells and caused a dose dependent cell death (Chen 2005). Furthermore, the authors showed that normal tissue cells, such as blood cells, are protected from the toxic effects of AA (Chen 2005).
A number of mechanisms have been proposed to understand how vitamin C functions in decreasing the viability of cancer cells. These include suppression of reactive oxygen species (ROS) on mitochondrial DNA, pro-oxidant function of vitamin C, inhibition of the nitric oxide (NO) pathway, degradation of hypoxia inducible factor-1 alpha (HIF-1 alpha), and maintenance of the extracellular matrix (Cha 2013, Du 2012, KC 2005, Mikirova 2008, Verrax 2008). The majority of in vitro and animal studies have focused on high dosage vitamin C acting as a pro-oxidant via the production of hydrogen peroxide (H2O2) (Chen 2008, Du 2012). By acting as an intermediate and readily donating electrons to redox-active transition metals including copper (Cu2+) or iron (Fe3+), AA reduces the transition metals to cuprous (Cu+) and ferrous (Fe2+) ions, respectively (Frei 2008). The reduced transition metals react with oxygen to produce superoxide radicals, which in turn, dismutate to form H2O2 and O2 (Frei 2008). Normal tissues have adequate blood flow which supplies anti-oxidant enzymes such as catalase and glutathione peroxidase to counter-harmful hydroxyl radicals (Ohno 2009). However, tumour cells have reduced blood flow which leads to an accumulation of H2O2 and subsequent apoptosis of these cells (Ohno 2009) (see Figure 2).
ROS can build up within cells due to intracellular oxidative events and can cause fragmentation of mitochondrial DNA (mtDNA) which is associated with a number of cancer types (Chatterjee 2006, KC 2005). AA has also been shown to protect mtDNA by directly reducing radical species and preventing the formation of reactive species, thereby inhibiting the action of ROS on nuclear DNA repair proteins (Padayatty 2003).
Hypoxia-inducible factor-1 (HIF-1) is a transcription factor that upregulates several tumour-growing mRNA transcripts (Verrax 2008). AA is a cofactor of proline hydroxylation and subsequently, the 26S proteasome degradation pathway of HIF-1, which consequently depletes necessary factors for tumour growth, resulting in growth suppression (Knowles 2003, Verrax 2008).
Another way that AA affects tumour cells is by inhibiting NO in endothelial cells (Mikirova 2008). The NO pathway has been known to be an important part of tumour angiogenesis and a high dose of AA has been observed to inhibit the production of NO, thereby decreasing blood vessel formation (Mikirova 2008) and preventing tumour growth (Folkmen 1971).
The breakdown of the extracellular matrix (ECM) has been implicated in the formation of tumours and metastasis and the aggressiveness of cancer growth (Cha 2013). Collagen makes up approximately one third of the ECM and serves as a physical barrier against invasion of cancerous cells (Du 2012, Egeblad 2010). AA is a nutrient required for the synthesis and assembly of collagen, protects against collagen degradation, and prevents proliferation of cancer (Cha 2013).
AA is also known to improve the sensitization of cancer cells to chemotherapy treatments (Heaney, 2008). Seven pathways or mechanisms have been identified to elucidate how AA enhances the effects of cancer therapy drugs. Many of these mechanisms involve the enhancement of membrane transport systems or the activation of tumour suppressor genes (Chiang 1994). As a result, AA reverses chemotherapyresistance and increases the delivery of chemotherapy into cancer cells, increases tumour cell membrane permeability to chemotherapy drugs, stabilizes p53 genes, inhibits translocation of NF-kB and AP1, inhibits Nrf2-mediated gene expression, and activates MLH1, c-Ab1 and p72-signalling cascades (Abdel-Latiff 2005, Bharti 2002, Chiang 1994, Dong 1993, Heaney, 2008, Tarumoto 2004).
In 2001, Reddy and colleagues showed that ascorbic acid stabilizes p53 genes Bax and Bcl2, involved in cancer progression. It has been shown that the absence of p53 leads to abolition of G1 arrest or apoptosis in response to ionizing radiation and DNA damaging agents (Reddy 2001). E6 is an oncoprotein that targets p53 and affect its cell-cycle regulation (Reddy 2001, Scheffner 1990). Reddy and colleagues (2001) showed that vitamin C causes a down-regulation of E6 which in turn causes the up-regulation of pro-apoptotic protein p53 and Bax as well as the down-regulation of apoptotic inhibitor Bcl-2. Accumulation of p53 and Bax then sensitizes the cells to cell-cycle arrest, cell death and apoptosis induced by chemotherapy agents such as cisplatin and etoposide (Reddy 2001).
Another proposed mechanism by which AA provides chemo-sensitization is through inhibition of nuclear factor-kB (NF-kB) and AP-1 (Abdel-Latiff 2005). NF-kB and AP-1 are nuclear transcription factors that have an effect on genes that promote cancer progression (Bharti 2002, Dong 1993). The former suppresses apoptosis and promotes tumourigenesis, while the latter activates genes involved in tumor promotion and progression (Bharti 2002, Dong 1993). Both transcription factors are activated in the presence of chemotherapy agents, thereby causing chemotherapy drug resistance (Abdel-Latiff 2005). Cell cultures studies have shown that AA helps to down-regulate the activity of NF-kB and AP-1, leading to reduced tumour cell proliferation and an improvement in the efficacy of chemotherapy drugs, though the exact mechanism of action of AA on these two factors has yet to be explored (Abdel-Latiff 2005).
Inhibit Nrf2-mediated gene expression
Ascorbic acid also inhibits Nrf2- mediated gene expression which results in greater sensitivity of cancerous cell to chemotherapeutic drugs. Nrf2 is a member of the family of basic regionleucine zipper transcription factors that migrates into the nucleus under conditions of oxidative stress (Tarumoto 2004). In the nucleus, Nrf2 regulates gene expression of a number of detoxifying enzymes such as -glutamylcysteine synthetase ( -GCS), the rate-limiting enzyme in glutathione (GSH) synthesis via the antioxidant response element (ARE)- mediated gene expression (Tarumoto 2004). GSH is involved in resistance to some anti-cancer drugs, including cisplatin and doxorubicin (Iida 1999). Ascorbic acid works to suppress the migration of Nrf2 to the nucleus, thus resulting in inhibition of GSH synthesis and restoration of sensitivity of cancerous cells to chemotherapy (Tarumoto 2004).
Lastly, ascorbic acid has been shown to activate MLH1, and p73 cascades. Mut L homologue-1 (MLH1) is a component of the DNA mismatch repair machinery which allows the removal of mismatches that occur during DNA replication, genetic recombination and DNA damage (Catani 2002). Defects in this gene lead to an accumulation of replication errors and can result in colorectal cancer (Peltomaki 2001). The response to DNA damage involves MLH1 which can activate c-Ab1 tyrosine kinase which then initiates the p53 homologue p73 which improves cellular susceptibility to apoptosis (Catani 2002). This susceptibility is crucial because chemotherapy agents work by causing DNA damage and depend on the cell’s apoptosis as a method of killing the tumour (Catani 2002).
PRE-CLINICAL STUDIES
Mouse Models
The ability of genetically engineered mice to mimic the pathophysiological and molecular features of human malignancies has allowed mouse models to become the most sophisticated animal model for human cancer (Frese 2007). A number of IVC studies have been conducted on mouse models in which decreased tumour growth and tumour volume were observed. However, since most of these studies did not investigate the effects of AA as a cotreatment to chemotherapy, the beneficial effects of AA may be underestimated.
A 1988 study by Tsao et al. reported that AA inhibited the growth of human mammary tumours in immunocompetent mice that were administered AA in drinking water and given a diet with cupric sulfate. AA as a dietary additive alone was ineffective, which supports the theory that the inhibitory effect of AA is due to H2O2 production as Cu2+ is known to catalyze the production of H2O2 in the presence of AA.
Following their in vitro study in 2005, Chen et al. in 2008 applied AA as a treatment option to ovarian, pancreatic and glioblastoma tumour xenografts in mice and found that a high dose of vitamin C (4g/ kg body weight), injected intraperitoneally, decreased tumour growth and weight of all three cancers in the range of 41-53% (Chen 2008). The authors attributed this reduction to H2O2 generation in the extracellular medium of tumour cells by the AA radical (Chen 2008).
Belin et al. (2009) conducted a study in which nude mice were injected with human colon adenocarcinoma cells and the treatment group was treated with high concentrations of AA (1000 mg/kg/day) while the control group received only 15/ mg/kg/day of AA. A decrease in tumour growth was only observed in the treatment group. Furthermore, four out of seven mice in the control group died during the course of the experiment. Thus, it was concluded that a high dose of AA given to cancerous mice will prevent invasive carcinogenic processes (Belin 2009).
Another study by Espey et al. (2011) applied IVC therapy in combination with gemcitabine, a chemotherapeutic agent used to treat pancreatic cancer. After implanting tumour cells into the flanks of mice, the authors divided the mice into four treatment groups: gemcitabine alone, AA alone, combination therapy and saline control (Espey 2011). They found that combination therapy was superior in reducing tumour volume and weight relative to gemcitabine treatment alone (Espey 2011). No adverse effects were recorded except for osmotic stress due to the sodium component of pharmacologic AA administration (Espey 2011).
Recently, Cha et al. (2013) used the collagen pathway to test the efficacy of AA on breast and melanoma cancer cell lines in knockout mice that are unable to synthesize AA. The mice were maintained on or deprived of AA in their diet for four weeks prior to and two weeks after intraperitoneal injections of melanoma and breast cancer cells (Cha 2013). AA supplemented mice showed a 28% tumour weight reduction as compared to the controls (Cha 2013). This provides further evidence that supplemented AA hinders metastasis, tumour growth and encapsulation of tumours (Cha 2013).
HUMAN TRIALS
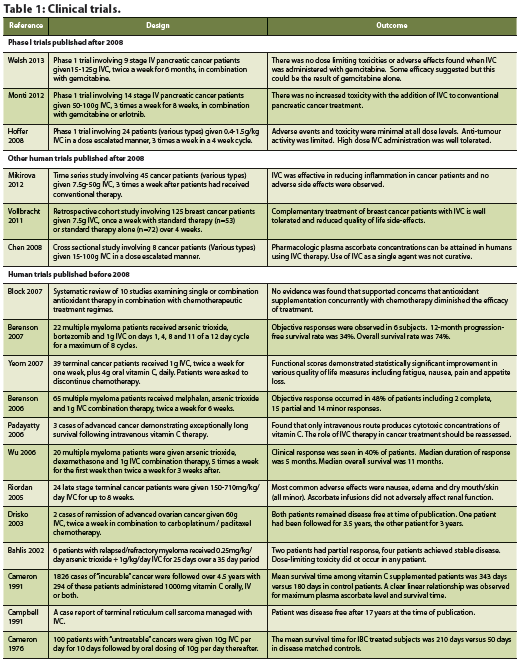
Disease Progression
There is limited evidence exploring the efficacy of chemotherapy plus IVC in humans. Since 2008, three phase I clinical trials have been conducted, which have demonstrated the safety of IVC alongside chemotherapy (Hoffer 2008, Monti 2012, Welsh 2013). A handful of human trials conducted before 2008 are also reviewed and included in Table 1. First, Hoffer et al. (2008) conducted a dose-seeking trial in patients with advanced tumour malignancy. The study results concluded that a safe dose of 1.5 g/kg of vitamin C can be used for future phase II trials and established that there were few adverse effects in both groups – with or without vitamin C administration (Hoffer 2008). The authors postulated that these adverse effects were related to the rapid infusion of a high osmolarity solution and could have been prevented by increased fluid intake (Hoffer 2008). With regards to the treatment, however, they reported no significant difference in life expectancy and no anti-tumour activity (Hoffer 2008).
The second phase I trial, conducted by Monti et al. (2012), aimed to establish safe levels of IVC administration alongside gemcitabine and erlotinib among patients with stage IV pancreatic cancer. The purpose was to evaluate whether the predicted AA levels could be achieved and to assess any response to the treatment (Monti 2012). The safety data did not reveal any adverse events irregular to the normal progression of pancreatic cancer or use of gemcitabine and erlotinib (Monti 2012).
Most recently, Welsh et al. (2013) aimed to establish the safety and tolerability of pharmacological AA alongside conventional gemcitabine in patients with stage IV pancreatic adenocarcinoma. Using disease burden, weight, performance status, time to progression, hematologic/metabolic tests and overall survival as outcome measures, the data suggested that pharmacologic vitamin C, alongside gemcitabine administration, is well tolerated.
Since these are phase I safety and dose-escalation trials, conclusions relating to the efficacy of vitamin C as a contributor to disease recession cannot be drawn. It should be noted that current phase I studies have only examined pancreatic cancer patients, which may not be representative of all cancer types. It is also important to note the lack of power when considering the conclusions of the aforementioned studies. Currently, the published evidence on the effectiveness of IVC treatment in conjunction with chemotherapy is limited to phase I clinical trials. There are three ongoing clinical trials investigating the safety of IVC therapy (phase I) (clin trials.gov NCT01833351), progressionfree survival (phase II) (clin trials.gov NCT01555489) and toxicity (phase II) (clin trials.gov NCT01754987), to provide better evidence for the role of AA in cancer therapy for the future.
Quality of Life
For patients facing the heavy disease burden of cancer and suffering from the side effects of treatment, improvement in quality of life may be equally as important as finding a cure. Several studies have suggested that IVC therapy may play a role in improving quality of life measures. A prospective study by Yeom et al. (2007) followed 39 terminal cancer patients over a one week period. Patients were asked to stop chemotherapy treatment and were administered 10g of vitamin C intravenously twice over the study period (Yeom 2007). Quality of life was assessed after the study period using a self-administered questionnaire. Patients reported a significant increase in overall quality of life as well as significant improvement in all areas of function (physical, role, emotional, cognitive and social).
Various symptoms were also improved including fatigue, nausea/vomiting, pain, sleep disturbance and appetite loss.
A retrospective cohort study conducted by Vollbracht et al. (2011) examined how IVC affected the quality of life of breast cancer patients. The study gathered 125 patients from different cancer clinics, of which 53 formed the study group and 72 formed the control group (Vollbracht 2011). Patients in the study group received 7.5g of IVC once a week for a minimum of four weeks along with standard antineoplastic interventions (surgical treatment, chemotherapy, radiotherapy or hormone therapy). The control group did not receive adjuvant IVC therapy. The main outcome measures include intensity of complaints due to disease or therapy during treatment and after care. After adjusting for age and baseline scores, the study found statistically significant results supporting the benefits of IVC on ameliorating side effects of treatment such as loss of appetite, fatigue, sleep disorders, and nausea (Vollbracht 2011). This study also reaffirmed the safety of IVC administration in conjunction with conventional cancer treatments.
For patients undergoing chemotherapy or radiation therapy, vitamin C may help to improve quality of life measures by increasing overall anti-oxidative capacity. Since mucosal and nerve cells are particularly susceptible to oxidative stress, increased vitamin C plasma levels may protect against gastrointestinal symptoms such as diarrhea (Vollbracht 2011). Vitamin C may also protect against the degradation of neurotransmitters commonly associated with oxidative stress, possibly explaining its antidepressant-like effects (Vollbracht 2011).
CONCLUSION:
Thus far, current data on the use of IVC as an adjunct therapy to conventional cancer treatments shows promise. While preclinical studies demonstrate the potential for AA to have cytotoxic effects on cancer cells via numerous pathways, hydrogen peroxide being the primary one, recent human trials have not yet proven these effects. However, IVC has been shown to improve the quality of life of cancer patients. Vitamin C’s relatively low-cost, easy accessibility and few treatment side effects further reinforce that its benefits should not be dismissed or overlooked. This review is a call for further research into the efficacy of conventional cancer treatment versus treatment in conjunction with IVC on a larger scale. Such data will help validate IVC as an integral component of standard cancer care and encourage physicians to consider this integrative treatment for their patients.
References
Abdel-Latiff MM, Raouf AA, Sabra K, Kelleher D, Reynolds JV. Vitamin C enhances chemosensitization of esophageal cancer cells in vitro. J Chemother. 2005 Oct;17(5):539-49.
Bahlis NJ, McCafferty-Grad J, Jordan-McMurry I, Neil J, Reis I, Kharfan-Dabaja M, Eckman J, Goodman M, Fernandez HF, Boise LH, Lee KP. Feasibility and correlates of arsenic trioxide combined with ascorbic acid-mediated depletion of intracellular glutathione for the treatment of relapsed/refractory multiple myeloma. Clin Cancer Res. 2002 Dec;8(12):3658-68.
Belin S, Kaya F, Duisit G, Giacomettei S, Ciccolini J, Fonte, M. Antiproliferative effect of ascorbic acid is associated with the inhibition of genes necessary to cell cycle progression. PLoS One. 2009 4(2):e4409.doi: 10.1371/journal.pone.0004409.
Berenson JR, Boccia R, Siegel D, et al. Efficacy and safety of melphalan, arsenic trioxide and ascorbic acid combination therapy in patients with relapsed or refractory multiple myeloma: a prospective, multicentre, phase II, single-arm study. Br J Haematol. 2006 Oct;135(2):174-83.
Berenson JR, Matous J, Swift RA, Mapes R, Morrison B, Yeh HS. A phase I/II study of arsenic trioxide/bortezomib/ ascorbic acid combination therapy for the treatment of relapsed or refractory multiple myeloma. Clin Cancer Res. 2007 Mar;13(6):1762-8.
Bharti AC, Aggarwal BB. Nuclear factor-kappa B and cancer: its role in prevention and therapy. Biochem Pharm 2002 Apr;12;64:883-888.
Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C. Impact of antioxidant supplementation on chemotherapeutic efficacy: a systematic review of the evidence from randomized controlled trials. Cancer Treat Rev. 2007 Sept;33(5):407-18.
Bram S, Froussard P, Guichard M, Jasmin C, Augery Y, Sinoussi- Barre F, & Wray W. Vitamin C preferential toxicity for malignant melanoma cells. Nature. 1980 Apr;17;284(5757):629-31.
Cameron E, Pauling L. (1978). Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4538-42.
Cameron E, Campbell A. Innovation vs. quality control: an ‘unpublishable’ clinical trial of supplemental ascorbate in incurable cancer. Med Hypotheses. 1991 Nov;36(3):185-9.
Campbell A, Jack T, Cameron E. Reticulum cell sarcoma: two complete ‘spontaneous’ regressions, in response to high-dose ascorbic acid therapy. A report on subsequent progress. Oncology. 1991;48(6):495-7.
Catani MV, Costanzo A, Savini I, Levrero M, De Laurenzi V, Wang, JYJ, Melino G, Avigliano L. Ascorbate up-regulates MLH1 (Mut L homologu-1) and p73: implications for the cellular response to DNA damage. Biochem J. 2002. Jun;364(2): 441-7
Cha J, Roomi MW, Ivanov V, Kalinovksy T, Niedzwieky A, Rath M. Ascorbate supplementation inhibits growth and metastasis of B16FO melanoma and 4T1 breast cancer cells in vitamin C-deficient mice. Int J Oncol. 2013 Jan;42(1):55-64. doi: 10.3892/ ijo.2012.1712.
Chatterjee A, Mambo E, Sidransky D. Mitochondrial DNA mutations in human cancer. Oncogene. 2006 Aug 7;25(34):4663-74.
Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl Acad Sci U S A. 2005 Sept;102(38):13604-9.
Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, Levine M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A. 2008 Aug;105(32):11105-9. doi: 10.1073/ pnas.0804226105
Chiang CD, Song EJ, Yang VC, Chao CC. Ascorbic acid increases drug accumulation and reverses vincristine resistance of human non-small-cell lung-cancer cells. Biochemical Journal. 1994 Aug 1;301(Pt 3), 759
Dong Z, Birrer MJ, Watts RG, Matrisian LM, Colburn NH. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proc Natl Acad Sci. 1994 Jan;91:609-613.
Drisko JA, Chapman J, Hunter VJ. The use of antioxidants with first-line chemotherapy in two cases of ovarian cancer. J Am Coll Nutr. 2003 Apr;22(2):118-23.
Du J, Cullen JJ, Buettner GR. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim Biophys Acta. 2012 Dec;1826(2):443-57. doi: 10.1016/j.bbcan.2012.06.003.
Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol. 2010 Oct;22(5):697-706. doi: 10.1016/j.ceb.2010.08.015.
Espey MG, Chen P, Chalmers B, Drisko J, Sun A, Levine M, Chen Q. Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic Biol Med. 2011 Jun;50(12):1726-7. doi: 10.1016/j.freeradbiomed.2011.03.030.
Frei B, Lawson S. Vitamin C and cancer revisited. Proc Natl Acad Sci U S A. 2008 Aug;105(32):11037-8 doi: 10.1073/ pnas.0806433105
Folkman J. Tumor angiogenesis: therapeutic implications. New England Journal of Medicine. 1971 Nov:285(21):1182-6. doi: 10.1056/NEJM197111182852108
Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007 Sept;7(9): 654–658. doi:10.1038/nrc2192
Heaney ML, Gardner JR, Karasavvas N, Golde DW, Scheinberg DA, Smith EA, O’Connor, OA. Vitamin C antagonizes the cytotoxic effects of antineoplastic drugs. Cancer research. 2008 Jul 25;68(19): 8031-8038.
Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, Miller WH. Phase I clinical trial of i.v.ascorbic acid in advanced malignancy. Ann Oncol. 2008 Nov;19(11):1969-74. doi: 10.1093/annonc/mdn377
Iida T, Mori E, Mori K, Goto S, Urata Y, Oka M, Kohno S, Kondo T. Co-expression of gamma-glutamycysteine synthetase subunits in response to cisplatin and doxorubicin in human cacner cells. Int J Cancer. 1999 Jul; 82(3): 405-11
KC S, Carcamo JM, Golde DW. Vitamin C enters mitochondria via facilitative glucose transporter 1 (GLUT1) and confers mitochondrial protection against oxidative injury. FASEB J. 2005 Oct;19(12):1657-67.
Knowles HJ, Raval RR, Harris AL, Ratcliffe PJ. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003;63:1764–1768.
Lemmo W. Enhancing chemotherapy effectiveness with simple vitamin C. Canadian Naturopathic Foundation. [Data file] Retried from http://www.exploreyourhealth.ca/viewpage. cfm?PageID=15
Mikirova NA, Ichim TE, Riordan NH. Anti-angiogenic effect of high doses of ascorbic acid. J Transl Med. 2008 Sep;6:50. doi: 10.1186/1479-5876-6-50.
Mikirova N, Casciari J, Rogers A, Taylor P. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J Transl Med. 2012 Sep;10:189. doi: 10.1186/1479-5876-10-189.
Monti DA, Mitchell E, Bazzan AJ, Littman S, Zabrecky G, Yeo CJ, Pillai MV, Newberg AB, Deshmukh S, Levine M. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One. 2012;7(1):e29794.
doi: 10.1371/journal. pone.0029794. Office of Dietary Supplements. Dietary Supplement Fact Sheet: Vitamin C. [Data file] Retrieved from http://ods.od.nih.gov/ factsheets/VitaminC-HealthProfessional/
Ohno S, Ohno Y, Suzuki N, Soma G, Inoue M. High-dose Vitamin C (Ascorbic Acid) Therapy in the treatment of patients with advanced cancer. Anticancer Res. 2009 Mar;29(3):809-15.
Padayatty SJ, Eck P, Kwon O, Lee J, Chen S, Corpe C, Dutta A, Dutta SK, Levine M. Vitamin C as an Antioxidant: Evaluation of Its role in Disease Prevention. J Am Coll Nutr. 2003 Feb;22(1):18- 35.
Padayatty SJ, Riordan HD, Hewitt SM, Katz A, Hoffer LJ, Lavine M. Intravenously administered vitamin C as cancer therapy: three cases. CMAJ. 2006 Mar;174(7):937-42.
Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann Intern Med. 2004 Apr;140(7):533-7.
Peltomaki P. Deficient DNA mismatch repair: a common etiologic factor for colon cancer. Hum Mol Genet. 2001 Apr; 10(7): 735-40
Reddy VG, Khanna N, Singh, N. Vitamin C augments chemotherapeutic response of cervical carcinoma HeLa cells by stabilizing p53. Biochem Biophys Res Commun. 2001 Mar;282(2): 409-15
Riordan HD, Casciari JJ, González MJ, Riordan NH, Miranda- Massari JR, Taylor P, Jackson JA.A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. P R Health Sci J. 2005 Dec;24(4):269-76.
Sakagami H, Satoh K, Hakeda Y, Kumegawa M. Apoptosisinducing activity of vitamin C and vitamin K. Cell Mol Biol (Noisy-le-grand). 2000 Feb;46(1):129-43.
Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec; 63(6):1129-36
Shah FD, Patel JB, Shukla SN, Shah PM, Patel PS. Evaluation of plasma non-enzymatic antioxidants in breast cancer etiology. Asian Pac J Cancer Prev. 2009 Jan-Mar;10(1):91-6.
Tarumoto T, Nagai T, Ohmine K, Miyoshi T, Nakamura M, Kondo T, Mitsugi K, Nakano S, Muroi K, Komatsu N, Ozawa K. Ascorbic acid restores sensitivity to imatinib via suppression of Nrf2-dependent gene expression in the imatinib-resistant cell line. Exp Hematol. 2004 Apr; 32(4); 375-81
Tsao SC, Dunham WB, Leung PY. In vivo antienoplastic activity of ascorbic acid for human mammary tumor. In Vivo. 1988 Mar- Apr;2(2):147-50.
Verrax J, Calderon PB. The controversial place of vitamin C in cancer treatment. Biochem Pharmacol. 2008 Dec;76(12):1644-52. doi: 10.1016/j.bcp.2008.09.024
Vollbracht C, Schneider B, Leendert V, Weiss G, Auerbach L, Beuth J. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: results of a retrospective, multicentre, epidemiological cohort study in Germany. In Vivo. 2011 Nov-Dec;25(6):983-90.
Welsh JL, Wagner BA, van’t Erve TJ, Zehr PS, Berg DJ, Halfdanarson TR, Yee NS, Bodeker KL, Du J, Roberts LJ 2nd, Drisko J, Levine M, Buettner GR, Cullen JJ. Pharmacological ascorbate with gemcitabine for the control of metastatic and nodepositive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother Pharmacol. 2013 Mar;71(3):765- 75. doi: 10.1007/s00280-013-2070-8.
Wu KL, Beksac M, van Droogenbroeck J, Amadori S, Zweegman S, Sonneveld P.Phase II multicenter study of arsenic trioxide, ascorbic acid and dexamethasone in patients with relapsed or refractory multiple myeloma. Haematologica. 2006 Dec;91(12):1722-3.
Yeom CH, Jung GC, Song KJ. Changes of terminal cancer patients’ health-related quality of life after high dose vitamin C administration. J Korean Med Sci. 2007 Feb;22(1):7-11.