Intravenous Therapies
Fish oil based lipid emulsions (FOBLE)
Fish oil based lipid emulsions (FOBLE) have an excellent record of safe and effective use, objectively evaluated in over 55 human intervention trials. Critical care hospital settings remain the most thoroughly evaluated areas of their application, however interest in management of flares of chronic inflammatory disorders such as arthritis and psoriasis has begun to emerge. By showcasing the clinical utility of this safe and effective intravenous treatment strategy, it is hoped, progress can be made in adding fish oil to the armament of intravenous therapies at the disposal of integrated healthcare providers.
Introduction
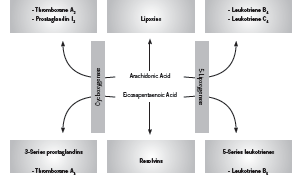
Fish derived omega-3 (n-3) fatty acids, specifically eicosapentanoic acid (EPA) and docosahexanoic acid (DHA) are well recognized for their anti-inflammatory effects (Rangel-Huerta 2012). Upon incorporation into the cell membrane, EPA and DHA competitively inhibit production of pro-inflammatory cytokines from arachidonic acid (AA), such as prostaglandin E2 (PGE2), and serve as a substrate for production of less active prostaglandin E3 (PGE3, anti-inflammatory) (Mayer 2006). In addition, EPA and DHA are precursors for the inflammation-resolving mediators appropriately known as resolvins (Calder 2010). The uses of intravenously (IV) administered fish oils, or fish oil based lipid emulsions (FOBLE), are lesser known. Nonetheless, there is a welldeveloped body of research demonstrating impressive clinical benefits associated with use of IV fish oils. The most supported application of IV fish oils is in the critical care setting, however other areas of application have also been investigated. Figure 1 shows the effect of EPA on generation of inflammatory cytokines.
A Pubmed search for “intravenous omega-3” on 10 August 2012 yielded 57 clinical trials. Not all of these are included here since some pertain to highly specialized applications, such as liver disease in premature infants, total parenteral (TPN) –related liver disease, endstage renal disease, or biomarker studies such as those investigating effects on antioxidant status. This article includes 18 clinical trials of intravenous omega-3 fatty acids related to the following areas: 1) critical care (n=13); 2) rheumatoid arthritis (n=2); and 3) psoriasis and inflammatory skin diseases (n=3).
Pharmacology
The most widely used and well-researched parenteral lipid emulsion featuring omega-3 fatty acids is Omegaven (Fresenius-Kabi, Germany). Omegaven is a 10% fish oil emulsion meaning that it contains 10g refined fish oil per 100mL, including between 1-3g each EPA and DHA (Calder 2010, Fresenius Kabi 2010). Other fish oil based formulations include SMOFLipid 20% (Fresenius-Kabi), which contains 30g total fish oils per 1000mL in combination with soybean oil, medium chain triglycerides, and olive oil (AusPAR 2010, Calder 2010); and Lipoplus (B. Braun, Germany), which contains 20g total fish oil per 1000mL in combination with soybean oil and medium chain triglycerides (B. Braun un-dated product information).
Intravenous delivery of n-3 PUFAs has been shown to circumvent the slower n-3 incorporation into phospholipid membranes following oral administration (Carpentier 2010, Roulet 1997, Simeons 2008). Incorporation of EPA into leukocyte and platelet membranes after IV administration occurs within 60 minutes (Carpentier 2010). Similarly, Madsen found that IV administration of 4.1g n-3 PUFAs (polyunsaturated fatty acids) resulted in an increase of levels present in platelet phospholipids at 4 hours, and increased levels in plasma phospholipids at 48 hours, while there was no change in the placebo group (2011).
A study by Roulet conducted among 10 postoperative patients found that a lipid emulsion with 10% fish oil added resulted in a greater than two-fold increase in the EPA content of platelet phospholipids, and decreased maximal platelet reaction speed (p <0.02) while increasing latency (p <0.002), indicating a less heightened immune response (1997). Importantly, no toxicities, including no increase in postoperative bleeding and no abnormalities in hepatic and renal function, were observed during the fish oil infusion (Roulet 1997). Pradier et al investigated the safety of a bolus IV injection of a medium-chain triglyceride:fish oil emulsion (8:2 ratio) on hemostatic parameters in 12 healthy subjects (2008). No adverse effect was found on 1) occlusion time in response to the ADP (adenosine diphosphate, a platelet activator) or the epinephrine test; 2) levels of certain markers of coagulability, such as fibrinogen, PAC-1, and others, in response to ADP, collagen or thrombin receptor analog peptide six when examined ex vivo. Authors concluded that these results support the hemostatic safety of the IV fish oil emulsion (Pradier 2008).
Clinical trials
Critical Care: Sepsis and Systemic Inflammatory Response Syndrome (SIRS) In the critical care setting, intravenous supplementation with fish oil is being studied for its powerful immunologic effects (Mayer 2006). In particular, IV fish oil has been shown to decrease the length of hospitalization in patients undergoing abdominal surgery (Jiang 2010), decrease levels of inflammation in patients with sepsis or SIRS (Sungertekin 2011), and decrease complications in post-operative patients (Heller 2006). For years, the standard lipid based emulsion used in patients requiring total or partial parenteral nutrition (TPN, PN) has consisted of soybean oil rich in omega-6 fatty acids (Mayer 2006). It was subsequently found however that high amounts of omega-6 fatty acids may in fact harmfully suppress immune function, resulting in increased rates of infection (Calder 2010, Nordenström 1979, Snydman 1982). Conversely, newer emulsions such as Omegaven containing 10% (w/v) fish oil have been shown to beneficially impact immune function in healthy (Pittet 2010, Pluess 2007) and hospitalized patients (Wei 2010).
In healthy patients, Omegaven has been shown to blunt the immune response to the endotoxin lipopolysaccharide (LPS) (Pittet 2010, Pluess 2007). Since bacterial-derived LPS acts as a trigger eliciting many of the harmful symptoms of infection and/ or sepsis essentially effected by the immune system, such as fever, systemic inflammation, and shock, reducing this reaction is considered beneficial in these patients. In addition, Pluess et al found that Omegaven blunted the effects of LPS on fever and the neuroendocrine response to infection (2007).
Patients
chain triglyceride; MCT medium chain triglyceride; NS non significant; PN parenteral nutrition; SICU surgical intensive
care unit; TPN total parenteral nutrition.
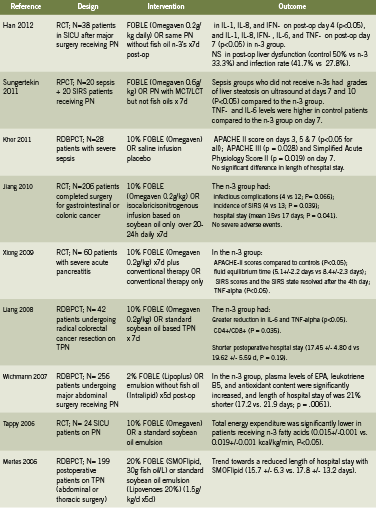
Table 1 presents a summary of 13 human trials investigating use of IV fish oil in critical care patients for outcomes related to sepsis, systemic inflammatory response syndrome (SIRS, syndrome secondary to severe infection), hospitalization, and mortality. These studies show that use of FOBLE may: improve post-operative liver function and rates of infection (Han 2012); improve Acute Physiology and Chronic Health Evaluation (APACHE) scores, a disease severity scoring system used in ICU settings, in patients with severe sepsis (Khor 2011) and pancreatitis (Xiong 2009); prevent infectious complications and incidence of SIRS, and reduce hospital stays in cancer patients undergoing major abdominal surgery (Jiang 2008, Liang 2008).
In addition, a 2010 meta analysis reviewed six RCTs conducted in Europe and Asia that compared parenteral nutrition with or without fish oil emulsion in postoperative patients (Wei 2010). Although in this study there was no significant impact on mortality, use of fish oil was associated with a significant reduction in infectious complications (relative risk RR 0.49, 95% confidence interval 0.26-0.93, P=0.03). The length of hospital stay was non-significantly decreased by over 3 days, with a decrease in 2.07 days in the intensive care unit (Wei 2010).
A prospective study of Omegaven among 661 patients with major abdominal surgery, abdominal sepsis, nonabdominal sepsis, serious trauma, or other diagnoses, and receiving TPN for three days or more, in 82 German hospitals, found that use of FOBLE resulted in: favorable effects on survival, infection rates, and length of stay, when administered in doses between 0.1 and 0.2 g/kg/day (Heller 2006). At doses of 0.15-0.2 g/kg/day, antibiotic requirements were 26% lower when compared with doses of <0.05 g/kg/day. After peritonitis and abdominal sepsis, the fish oil dose for minimizing length of intensive care unit stay was 0.23 g/kg/day (Heller 2006).
Rheumatoid Arthritis
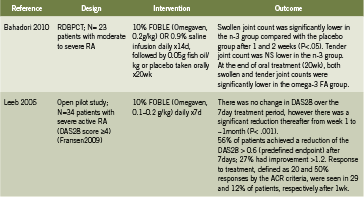
Table 2 summarizes two human trials investigating IV fish oil emulsions in the treatment of active rheumatoid arthritis (RA) (Bahadori 2010, Leeb 2006). In a randomized, double blind, placebo controlled trial, Bahadori administered IV fish oil 0.2g/kg daily for 14 days in patients with moderate to severe RA, followed by oral fish oil for 20 weeks, and found that after only one week, as well as after two weeks, swollen joint count was significantly lower in the fish oil group (2010). This effect persisted to the end of 20 weeks/ end of oral supplementation as well. Leeb conducted an open pilot trial in patients with severe RA, administering 0.1-0.2g/ kg fish oil daily for seven days; there was a significant reduction in disease severity ratings over time (p<0.001) and tolerability was rated as “excellent” (Leeb 2006). Intravenous fish oil may represent a safe and rapidly acting strategy to control severe acute RA.
Dermatology: Psoriasis and Atopic Dermatitis Table 3 summarizes three human trials investigating IV fish oil emulsions for the treatment of psoriasis or atopic dermatitis (Grimminger 1993, Mayser 2002, Mayser 1998). In patients with moderate to severe atopic dermatitis, IV fish oil for 10 days resulted in significant improvement in disease severity (visible by day 6) (p<0.05) compared to placebo (Mayser 2002). An earlier study by the same team examined patients hospitalized for chronic plaque type psoriasis (Mayser 1998). Treatment with Omegaven for 14 days resulted in significant reduction in the Psoriasis Activity Severity Index (PASI) (p=0.048) in the fish oil group compared to controls. A total of 16 of 43 patients (37%) in the fish oil group experienced a clinical response, defined as a reduction in PASI of 50% or greater, compared to 23% in the control group.
Finally, Grimminger found that IV fish oil improved disease severity between 45- 76% depending on the scoring measure (p<0.05 for all) in hospitalized patients with psoriasis involving 10% or more of their body surface area (1993). Remarkably, the treatment effect was evident within four to seven days of daily IV fish oil administration. Finally, IV fish oils have been shown to reduce cardiac arrhythmias (Heidt 2009) and improve PN-associated liver disease (Le 2010), however these applications are beyond the scope of this paper.
Conclusion
Intravenously administered fish-derived omega-3 fatty acids have been studied for a number of indications including the treatment and prevention of serious infection and SIRS in hospitalized patients; treatment of acute rheumatoid arthritis; inflammatory skin conditions including psoriasis and atopic dermatitis; as well as the prevention of arrhythmias and liver disease. In these populations, IV fish oil has been demonstrated to improve immune function, shorten hospitalization, reduce mortality, and significantly decrease disease activity. In some cases, the rate of disease improvement is rapid, occurring within the first week of treatment. Although parenteral fish oil emulsions are not currently available to NDs, we hope that access to this safe and efficacious agent will be broadened in the future
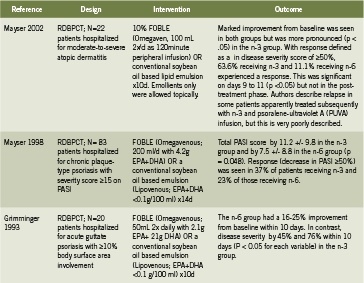
Key: PASI Psoriasis Area and Severity Index; RCT randomized controlled trial; RDBPCT randomized double blind
placebo controlled trial;
References
Antébi H, Mansoor O, Ferrier C, Tétégan M, Morvan C, Rangaraj J, Alcindor LG. Liver function and plasma antioxidant status in intensive care unit patients requiring total parenteral nutrition: comparison of 2 fat emulsions. JPEN J Parenter Enteral Nutr. 2004 May-Jun;28(3):142-8.
AusPAR: Australian Public Assessment Report for SMOF Lipid. Product Information. June 2010. www.tga.gov.au/pdf/auspar/ausparsmoflipid. pdfAccessed 29 August 2012.
Bahadori B, Uitz E, Thonhofer R, Trummer M, Pestemer-Lach I, McCarty M, Krejs GJ. Omega-3 Fatty acids infusions as adjuvant therapy in rheumatoid arthritis.JPEN J Parenter Enteral Nutr. 2010 Mar-Apr;34(2):151-5.
B. Braun. Clinical Nutrition Short Guide, Lipoplus Product Information. http://www.bbraun.com/documents/Knowledge/Short_ Guide_Clinical_Nutrition.pdf Accessed 29 August 2012.
Calder PC, Jensen GL, Koletzko BV, Singer P, Wanten GJ. Lipid emulsions in parenteral nutrition of intensive care patients: current thinking and future directions. Intensive Care Med. 2010 May;36(5):735-49.
Calder PC. Hot topics in parenteral nutrition. Rationale for using new lipid emulsions in parenteral nutrition and a review of the trials performed in adults. ProcNutr Soc. 2009 Aug;68(3):252-60.
Carpentier YA, Hacquebard M, Portois L, Dupont IE, Deckelbaum RJ, Malaisse WJ. Rapid cellular enrichment of eicosapentaenoate after a single intravenous injection of a novel medium-chain triacylglycerol:fish-oil emulsion in humans. Am J ClinNutr. 2010 Apr;91(4):875-82.
de Meijer VE, Gura KM, Meisel JA, Le HD, Puder M. Parenteral fish oil monotherapy in the management of patients with parenteral nutrition-associated liver disease. Arch Surg. 2010 Jun;145(6):547-51.
Fallon EM, Le HD, Puder M. Prevention of parenteral nutritionassociated liver disease: role of omega-3 fish oil. CurrOpin Organ Transplant. 2010 Jun;15(3):334-40.
Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Rheum Dis Clin North Am. 2009 Nov;35(4):745- 57, vii-viii.
Fresenius Kabi New Zealand Ltd. Consumer Medicine Information for Omegaven. July 2010. www.medsafe.govt.nz/consumers/cmi/o/ omegaven.pdfAccessed 29 August 2012.
Grimm H, Mertes N, Goeters C, Schlotzer E, Mayer K, Grimminger F, Fürst P. Improved fatty acid and leukotriene pattern with a novel lipid emulsion in surgical patients. Eur J Nutr. 2006 Feb;45(1):55-60.
Grimminger F, Mayser P, Papavassilis C, Thomas M, Schlotzer E, Heuer KU, Führer D, Hinsch KD, Walmrath D, Schill WB, et al. A double-blind, randomized, placebo-controlled trial of n-3 fatty
acid based lipid infusion in acute, extended guttate psoriasis. Rapid improvement of clinical manifestations and changes in neutrophil leukotriene profile.ClinInvestig. 1993 Aug;71(8):634-43.
Han YY, Lai SL, Ko WJ, Chou CH, Lai HS.Effects of fish oil on inflammatory modulation in surgical intensive care unit patients. NutrClinPract. 2012 Feb;27(1):91-8.
Heidt MC, Vician M, Stracke SK, Stadlbauer T, Grebe MT, Boening A, Vogt PR, Erdogan A. Beneficial effects of intravenously administered N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a prospective randomized study. ThoracCardiovasc Surg. 2009 Aug;57(5):276-80.
Heller AR, Rössler S, Litz RJ, Stehr SN, Heller SC, Koch R, Koch T. Omega-3 fatty acids improve the diagnosis-related clinical outcome. Crit Care Med. 2006 Apr;34(4):972-9.
Jiang ZM, Wilmore DW, Wang XR, Wei JM, Zhang ZT, Gu ZY, Wang S, Han SM, Jiang H, Yu K. Randomized clinical trial of intravenous soybean oil alone versus soybean oil plus fish oil emulsion after gastrointestinal cancer surgery. Br J Surg. 2010 Jun;97(6):804-9.
Khor BS, Liaw SJ, Shih HC, Wang LS. Randomized, double blind, placebo-controlled trial of fish-oil-based lipid emulsion infusion for treatment of critically ill patients with severe sepsis. Asian J Surg. 2011 Jan;34(1):1-10.
Le HD, de Meijer VE, Zurakowski D, Meisel JA, Gura KM, Puder M. Parenteral fish oil as monotherapy improves lipid profiles in children with parenteral nutrition-associated liver disease. JPEN J Parenter Enteral Nutr. 2010 Sep-Oct;34(5):477-84.
Leeb BF, Sautner J, Andel I, Rintelen B. Intravenous application of omega-3 fatty acids in patients with active rheumatoid arthritis. The ORA-1 trial.An open pilot study. Lipids. 2006 Jan;41(1):29-34.
Liang B, Wang S, Ye YJ, Yang XD, Wang YL, Qu J, Xie QW, Yin MJ. Impact of postoperative omega-3 fatty acid-supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients.World J Gastroenterol. 2008 Apr 21;14(15):2434-9.
Madsen T, Christensen JH, Thøgersen AM, Schmidt EB, Toft E. Intravenous infusion of n-3 polyunsaturated fatty acids and inducibility of ventricular tachycardia in patients with implantable cardioverter defibrillator. Europace. 2010 Jul;12(7):941-6.
Madsen T, Christensen JH, Toft E, Aardestrup I, Lundbye- Christensen S, Schmidt EB. Effect of intravenous omega-3 fatty acid infusion and hemodialysis on fatty acid composition of free fatty acids and phospholipids in patients with end-stage renal disease. JPEN J Parenter Enteral Nutr. 2011 Jan;35(1):97-106.
Mayer K, Schaefer MB, Seeger W. Fish oil in the critically ill: from experimental to clinical data. CurrOpinClinNutrMetab Care. 2006 Mar;9(2):140-8.
Mayer K, Gokorsch S, Fegbeutel C, Hattar K, Rosseau S, Walmrath D, Seeger W, Grimminger F. Parenteral nutrition with fish oil modulates cytokine response in patients with sepsis. Am J RespirCrit Care Med. 2003 May 15;167(10):1321-8. A
Mayer K, Fegbeutel C, Hattar K, Sibelius U, Krämer HJ, Heuer KU, Temmesfeld-Wollbrück B, Gokorsch S, Grimminger F, Seeger W. Omega-3 vs. omega-6 lipid emulsions exert differential influence on neutrophils in septic shock patients: impact on plasma fatty acids and lipid mediator generation. Intensive Care Med. 2003 Sep;29(9):1472-81. B
Mayser P, Mayer K, Mahloudjian M, Benzing S, Krämer HJ, Schill WB, Seeger W, Grimminger F. A double-blind, randomized, placebocontrolled trial of n-3 versus n-6 fatty acid-based lipid infusion in atopic dermatitis. JPEN J Parenter Enteral Nutr. 2002 May- Jun;26(3):151-8.
Mayser P, Mrowietz U, Arenberger P, Bartak P, Buchvald J, Christophers E, Jablonska S, Salmhofer W, Schill WB, Krämer HJ, Schlotzer E, Mayer K, Seeger W, Grimminger F. Omega-3 fatty acid-based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am AcadDermatol. 1998 Apr;38(4):539-47.
Mertes N, Grimm H, Fürst P, Stehle P. Safety and efficacy of a new parenteral lipid emulsion (SMOFlipid) in surgical patients: a randomized, double-blind, multicenter study. Ann NutrMetab. 2006;50(3):253-9.
Park KT, Nespor C, Kerner J Jr. The use of Omegaven in treating parenteral nutrition-associated liver disease. J Perinatol. 2011 Apr;31Suppl 1:S57-60.
Pluess TT, Hayoz D, Berger MM, Tappy L, Revelly JP, Michaeli B, Carpentier YA, Chioléro RL. Intravenous fish oil blunts the physiological response to endotoxin in healthy subjects. Intensive Care Med. 2007 May;33(5):789-97.
Nordenström J, Jarstrand C, Wiernik A. Decreased chemotactic and random migration of leukocytes during Intralipid infusion. Am J Clin Nutr. 1979 Dec;32(12):2416-22.
Pradier O, Portois L, Malaisse WJ, Carpentier YA. Hemostatic safety of the bolus intravenous injection of a novel medium-chain triglyceride:fish oil emulsion. Int J Mol Med. 2008 Sep;22(3):301-7.
Rangel-Huerta OD, Aguilera CM, Mesa MD, Gil A. Omega-3 longchain polyunsaturated fatty acids supplementation on inflammatory biomakers: asystematic review of randomised clinical trials. Br J Nutr. 2012 Jun;107Suppl2:S159-70.
Roulet M, Frascarolo P, Pilet M, Chapuis G. Effects of intravenously infused fish oil on platelet fatty acid phospholipid composition and on platelet function in postoperative trauma. JPEN J Parenter Enteral Nutr. 1997 Sep-Oct;21(5):296-301.
Schrepf R, Limmert T, Claus Weber P, Theisen K, Sellmayer A. Immediate effects of n-3 fatty acid infusion on the induction of sustained ventricular tachycardia. Lancet. 2004 May 1;363(9419):1441-2.
Simoens CM, Deckelbaum RJ, Massaut JJ, Carpentier YA. Inclusion of 10% fish oil in mixed medium-chain triacylglycerol-long-chain triacylglycerol emulsions increases plasma triacylglycerol clearance and induces rapid eicosapentaenoic acid (20:5n-3) incorporation into blood cell phospholipids. Am J ClinNutr. 2008 Aug;88(2):282-8.
Snydman DR, Murray SA, Kornfeld SJ, Majka JA, Ellis CA. Total parenteral nutrition-related infections. Prospective epidemiologic study using semiquantitative methods. Am J Med. 1982 Nov;73(5):695-9. -Omega-6 is not mentioned anywhere in this article
Sungurtekin H, Değirmenci S, Sungurtekin U, Oguz BE, Sabir N, Kaptanoglu B. Comparison of the effects of different intravenous fat emulsions in patients with systemic inflammatory response syndrome and sepsis. NutrClinPract. 2011 Dec;26(6):665-71.
Tappy L, Berger MM, Schwarz JM, Schneiter P, Kim S, Revelly JP, Chioléro R. Metabolic effects of parenteral nutrition enriched with n-3 polyunsaturated fatty acids in critically ill patients.ClinNutr. 2006 Aug;25(4):588-95.
Tomsits E, Pataki M, Tölgyesi A, Fekete G, Rischak K, Szollár L. Safety and efficacy of a lipid emulsion containing a mixture of soybean oil, medium-chain triglycerides, olive oil, and fish oil: a randomised, double-blind clinical trial in premature infants requiring parenteral nutrition. J PediatrGastroenterolNutr. 2010 Oct;51(4):514-21.
Wei C, Hua J, Bin C, Klassen K. Impact of lipid emulsion containing fish oil on outcomes of surgical patients: systematic review of randomized controlled trials from Europe and Asia. Nutrition. 2010 May;26(5):474-81.
Wichmann MW, Thul P, Czarnetzki HD, Morlion BJ, Kemen M, Jauch KW. Evaluation of clinical safety and beneficial effects of a fish oil containing lipid emulsion (Lipoplus, MLF541): data from a prospective, randomized, multicenter trial. Crit Care Med. 2007 Mar;35(3):700-6.
Xiong J, Zhu S, Zhou Y, Wu H, Wang C. Regulation of omega-3 fish oil emulsion on the SIRS during the initial stage of severe acute pancreatitis. J HuazhongUnivSciTechnolog Med Sci. 2009 Feb;29(1):35-8.