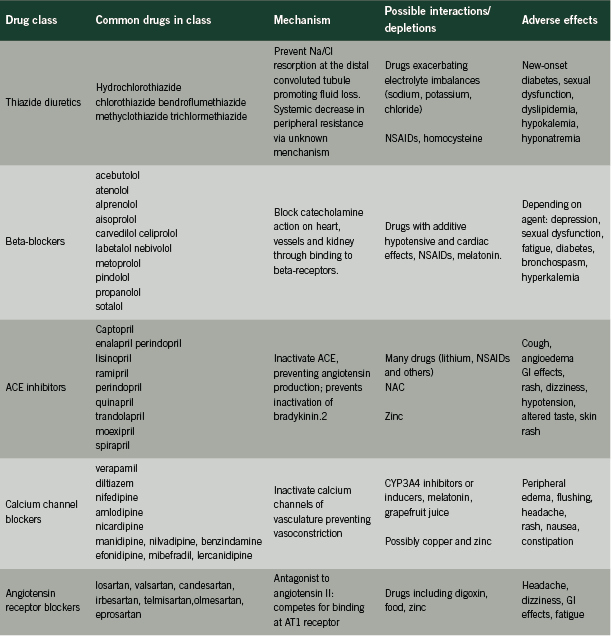
First- line antihypertensives
Mechanisms of action, adverse effects and potential interactions
Hypertension is estimated to affect 23% of the Canadian population, or approximately six million adults. Prevalence increases with age: more than 70% of adults over the age of 80 are affected by this condition (Robitaille 2011).
Elevated blood pressure is a well-established risk factor for cardiovascular events including myocardial infarction and stroke (Leone 2011). Chronic kidney disease and other conditions are complicated and exacerbated by hypertension (Koizumi 2011). In light of the risks associated with this presentation, blood pressure reduction is an important therapeutic goal.
Although lifestyle changes including weight reduction, exercise prescriptions and diet modifications are primary recommendations in sustainable hypertension management, pharmacological therapies are commonly used. Current recommendations from the Canadian Hypertension Education Program (CHEP) indicate that first-line drug therapy should consist of one of the following agents:
• A thiazide diuretic;
• A beta-blocker;
• An angiotensin-converting enzyme inhibitor (ACE inhibitors);
• A long-acting calcium channel blocker (CCB); or
• An angiotensin receptor blocker (ARB). Should monotherapy be ineffective, a combination of select agents is encouraged (Rabi 2011).
This review will examine these five classes of drugs, describing their mechanism of action, adverse effects and potential interactions with drugs, nutrients and endogenous substances.
Mechanisms of blood pressure regulation
Adequate circulatory pressure ensures appropriate perfusion of all tissues of the body, supporting the delivery of oxygen, nutrients and other essential factors to all cells. The crucial task of blood pressure regulation relies upon the interplay of numerous physiologic systems.
The renin-angiotensin-aldosterone system (RAAS) is integral to the dynamics of blood pressure regulation (Crowley 2007) and has a powerful influence upon vascular tone and fluid volume. This system is the target of ACE inhibitors and ARBs.
Renin, a proteolytic enzyme, is central to the RAAS. Once secreted by the juxtaglomerular cells of the kidneys, renin cleaves inactive angiotensinogen circulating in the blood. Angiotensin I is formed and converted in turn to angiotensin II by angiotensin-converting enzyme (ACE) (Raebel 2011). Angiotensin II goes on to stimulate angiotensin 1 receptors (AT1) in the heart, blood vessels, kidney, adrenals and brain, causing vasoconstriction, vasopressin and aldosterone release, sodium retention and increased sympathetic activity (Burnier 2001, Raebel 2011). Systemic blood volume and pressure increase as a result.
Interactions between ACE and bradykinin further increase circulatory pressure. Bradykinin promotes vasodilation through nitric oxide release while encouraging salt loss through the urine (Piepho 2000); ACE inactivates bradykinin through a cleaving process.
Calcium dynamics are important to the maintenance of vascular tone (Richard 2005). Influx of calcium across cell membranes causes contraction of smooth muscle cells lining the circulatory system, resulting in vasoconstriction and an increase in blood pressure. This mechanism is the therapeutic target of calcium channel blockers (CCBs).
The sympathetic nervous system can induce changes in vascular tone, renin levels and cardiac function. Adrenergic receptors in numerous organs and tissues respond to catecholamine release causing:
• increased heart rate and output (through beta-1 receptors);
• vasoconstriction and vasodilation (through alpha-2 and beta-2 receptors); and
• increased renin production (through beta-1 receptors).
Beta-type adrenoreceptor blocking drugs (beta-blockers) act upon these receptors with varying degrees of selectivity (Gorre 2010).
Finally, appropriate blood pressure levels require adequate intravascular fluid. Complex neurohormonal mechanisms involving the kidneys, the thirst response, aldosterone and anti-diuretic hormone (ADH) ensure that fluid is conserved and excreted from the body as needed (Thornton 2010).Thiazide diuretics influence this system, promoting fluid loss and decreasing blood pressure.
THIAZIDE DIURETICS
Thiazide diurectics have been used in the management of hypertension for over 50 years (Sarafidis 2010). Still considered a first-line agent, thiazide diuretics appear to be more beneficial than other anti-hypertensives in their ability to reduce cardiovascular morbidity and mortality (Wright 2009). Thiazide diuretics have also been shown to be more effective than ACE inhibitors or ARBs in patients of African descent (Taylor 2005).
Mechanism of action
Thiazide diuretics act at the distal convoluted tubule of the nephron, inhibiting the activity of the NaCl co-transport pathway (Sarafidis 2010). The action of these drugs prevents the resorption of sodium and chloride at this site and promotes fluid loss through the urine.
Paradoxically, fluid levels return to pre-treatment levels within four to six weeks of treatment initiation, yet pressure decrease is maintained. This hypotensive effect may be explained through the apparent ability of thiazide diuretics to reduce peripheral vascular resistance and blood pressure, but the mechanism is not understood at present (Duarte 2010).
Adverse effects
Higher levels of sodium in the collecting duct of the nephron result in increased potassium loss, creating the potential for both hyponatremia (Egom 2011) and hypokalemia. Potassium loss can increase the risk of sudden cardiac death although the use of lower-dose regimens and concurrent ACE inhibitors may attenuate this risk (Krämer 2000). Hypokalemia is cited as a possible cause of new-onset diabetes associated with thiazide diuretic use (Sica 2011). Magnesium loss (Sarafidis 2010) and uric acid retention (Sica 2011) are also associated with thiazide diuretic use.
Erectile dysfunction has been reported in men using thiazide diuretics (Francis 2007). Dyslipidemia has also been attributed to thiazide use, but newer research suggests inconsistent effects upon lipid levels in the absence of increased cardiovascular risk (Deano 2012).
Potential interactions
Care should be taken with any drugs that decrease potassium, magnesium or sodium levels as an additive effect may result. The hypotensive effect of thiazide diuretics may be decreased with NSAID use (Sica 2011). Digoxin intoxication has been associated with concomitant diuretic use (Wang 2010).
Plasma homocysteine levels may be increased with thiazide diuretic use (Westphal 2003). Additional monitoring of homocysteine levels may be necessary in these patients.
BETA-BLOCKERS
Beta-blockers have been used since the mid-1960’s in the management of cardiovascular conditions (Chrysant 2008). This diverse class includes both lipophilic and hydrophilic drugs, varying in their receptor selectivity and duration of effect. Beta-blockers continue to be considered first line agents in the management of hypertension (Rabi 2011) despite recent criticisms of their ability to prevent stroke and other negative outcomes in comparison to other anti-hypertensives (Wiysonge 2007).
Mechanism of action
Beta-blockers exert their influence by competing with catecholamines for absorption by beta-1 adrenergic receptors. This binding blocks the effect of endogenous neurotransmitters, decreasing the effects of the sympathetic nervous system causing reduced blood pressure, heart rate and cardiac contractility. Non-selective beta-blockers such as propanolol may also act upon beta-2 receptors in the lungs, causing undesirable pulmonary effects in some individuals (van der Woude 2005). Newer agents (carvedilol and nebivolol) promote additional vasodilation through nitric oxide production (Chrysant 2008).
Adverse effects
Depression associated with beta-blocker prescription has been reported in the literature (Patten 1990) although much current research does not support this association (Ko 2002, Verbeek 2011). Others suggest that while depressive symptoms may occur with beta-blockers use, the effect is limited to lipophilic agents (propanolol, alprenolol) that can cross the blood-brain barrier (Luijendijk 2011).
Sexual dysfunction and fatigue have been associated with beta-blocker use (Ko 2002) but may again be related to the specific agents used (Brixius 2007). In addition, some beta-blockers may increase the risk of developing new-onset diabetes (Gorre 2010). It is unclear whether newer vasodilating agents such as carvedilol, nebivolol and labetolol share these properties with older agents (Ram 2010).
Non-selective beta-blockers have been contraindicated in patients with lung disorders as inhibition of the beta-2 adrenoreceptors in the lungs may cause bronchospasms in these patients (Kendall 1997). Newer drugs that are selective for beta-1 receptors have not been found to cause the same issues and appear to be safe for use in patients with pulmonary concerns (Salpeter 2005).
Like ACE inhibitors and ARBs discussed below, beta-blockers increase the risk of hyperkalemia as a result of decreased renin production (Takaichi 2007).
Potential interactions
Care should be taken with concomitant use of any other drugs with hypotensive or negative inotropic or chronotropic effects. Interactions between beta-blockers and NSAIDs such as ibuprofen have also been reported in the literature (Salort- Llorca 2008) resulting in decreased effectiveness of this antihypertensive medication.
Melatonin production appears to be impaired by some beta-blockers (Stoschitzky 1999) with the notable exception of carvedilol. Melatonin supplementation may be indicated in patients treated with drugs from this class (Fares 2011).
ACE INHIBITORS
ACE inhibitors form a diverse group of drugs that inhibit the RAAS through their action upon angiotensin-converting enzyme (ACE).
Mechanism of action
ACE inhibitors bind to ACE through the formation of zinc ligands (Piepho 2000). The production of angiotensin II is thereby impaired, preventing AT1-mediated increases in vascular tone, aldosterone production and bradykinin degradation (Hayduk 1999). The RAAS is essentially inactivated, preventing further increases in blood pressure.
Adverse effects
The most common adverse effect associated with ACE inhibitor use is a dry cough (Semple 1995) that while not serious, can prompt treatment discontinuation. Cough is believed to be associated with an accumulation of bradykinin secondary to ACE inhibition (Mas 2011).
Patients using ACE inhibitors are also at risk of hyperkalemia (Raebel 2011), resulting from the ACE-mediated decrease in aldosterone production (Piepho 2000). This adverse effect may be more pronounced with concomitant renal or cardiac failure (Izzo 2011). ACE inhibitors are not recommended in patients with renal stenosis (Parish 1992). Other reported adverse effects include angioedema (Izzo 2011), rash, dizziness, gastrointestinal (GI) effects, hypotension and altered taste (Parish 1992).
The safety of ACE inhibitors has not been clearly established in pregnancy. Although significant adverse effects to the fetus have not been seen in some trials (Diav-Citrin 2011), this class of drugs is generally regarded as contraindicated during pregnancy (Cooper 2006, Izzo 2011).
Potential interactions
Many drug-drug interactions are possible with ACE inhibitor use (Hines 2011). Lithium toxicity is possible with concurrent ACE inhibitor use (Piepho 2000) and NSAIDs can decrease the anticipated hypotensive effect of this intervention (Piepho 2000, Salort-Llorca 2008).
A small crossover study found that N-acetyl cysteine (NAC) may potentiate the hypotensive effect of captopril and enalapril (Barrios 2002). This may be a desirable effect in some patients. Endogenous zinc levels may be depleted by ACE inhibitor use (Golik 1998)
CALCIUM-CHANNEL BLOCKERS (CCBs)
CCBs have been in use for half a century (Nayler 1986) and have evolved through several generations of drugs from short-acting drugs (verapamil), through longer-acting drugs (nisoldipine), to newer, slower-release drugs with great affinity for their designated receptors (manidipine, lercanidipine) (Richard 2005).
Mechanism of action
Contraction of muscular tissue lining the circulatory system is mediated in part by intracellular calcium levels. CCB-type drugs bind to the calcium channels that govern the flow of calcium across cellular membranes and inactivate them, preventing the influx of calcium and subsequent contraction of vascular tissue. (Richard 2005). Some CCBs may also decrease heart rate and contractility, especially first-generation CCBs (Noll 1998). Blood pressure is decreased as a result.
Numerous sub-types of calcium channels have been identified. L-type channels are dominant through the vascular system and are the target of dihydropyridine CCBs such as amlodipine and nicardipine. Newer agents including manidipine, benzindamine and nilvadipine may act upon T-type channels found in the kidneys, conferring renal protection in comparison to older drugs. Other channels (P/Q type and others) are being investigated as potential therapeutic targets (Hansen 2011).
Adverse effects Peripheral dependent edema is a significant side effect, especially with the use of first- and second-generation CCBs (Burnier 2009). Earlier concerns with increased risk of MI seem to have been associated with large doses of shorter-acting agents rather than drugs in current use (Kaplan 1996). Headaches and flushing are common effects (Makarounas-Kirchmann 2009) in addition to rash, nausea and drowsiness. High-dose verapamil may cause constipation (Elliott 2011).
Calcium channel blockers are used in pregnancy, but a recent retrospective study found an increased risk of infant seizures with pre-term CCB exposure (Davis 2011).
Potential interactions
Plasma levels of cyclosporine and digoxin may be increased by diltiazem and verapamil (Elliott 2011). Many other potential drug-drug interactions exist between CCBs and inhibitors and inducers of CYP3A4. For this reason, CCBs should not be taken with grapefruit juice (Sica 2006a). A recent trial (Koziróg 2011) demonstrated reduced efficacy of nifedipine when used in conjunction with melatonin. Competition for common binding sites may explain this interaction. This drug may also decrease copper levels and increase zinc levels (Misiewicz 1998) but the magnitude and clinical significance of this effect has not been established.
ANGIOTENSIN RECEPTOR BLOCKERS (ARBs)
ARBs are comparatively new additions to the spectrum of hypertensive drugs, with losartan, the first drug in this class, being introduced in 1995 (Sica 2006). Their efficacy appears to be comparable to that of ACE inhibitors (Matchar 2008).
Mechanism of action
Like ACE inhibitors, ARBs affect the RAAS directly, preventing angiotensin II from exerting pressure-increasing effects upon the circulatory system. ARBs are angiotensin II antagonists, binding competitively to type 1 angiotensin receptors and decreasing blood pressure as a result. Some decreases in plasma aldosterone levels may also be anticipated through this effect (Sica 2006).
Adverse effects
In comparison to other antihypertensive agents, ARBs appear to have fewer associated adverse effects (Matchar 2008) and are generally well-tolerated. Notably, ARBs are not associated with a dry cough, unlike ACE inhibitors (Fogari 2011). GI disturbance, fatigue, dizziness, headache and hyperkalemia may present with ARB use (Fogari 2011, Raebel 2011).
Some concerns have been raised recently regarding associations between ARBs and cancer, based upon both meta-analyses (Bangalore 2011, Sipahi 2010) and population studies (Chang 2011). Others have not found evidence of any risk with ARB use (Yoon 2011). Current recommendations advise continued use of ARBs pending further investigation of this link, citing established benefits to cardiovascular health and a lack of conclusive evidence of risk (Rabi 2011, Volpe 2011).
Populations that should not use ARBs include individuals with kidney disease (Sipahi 2010). Acute renal failure may result from use by individuals with renal stenosis (Burnier 2001). ARBs are also not recommended for use in pregnancy (Alwan 2005) although small studies have not shown detriment from early exposure (Diav-Citrin 2011).
Potential interactions
Increased digoxin levels (Elliott 2006) have been reported with ARB use. Other drug-drug interactions are possible. ARBs including valsartan and eprosartan should not be taken with food as this may decrease absorption by up to 40% (Burnier 2001). Like other hypertensive agents, ARBs appear to interact with zinc, promoting excretion and potential deficiency (Koren- Michowitz 2005).
CONCLUSION
Current guidelines encourage the use of pharmacological therapy in the management of hypertension using drugs from the five classes described above. A thorough understanding of mechanisms, interactions and potential adverse effects will encourage safe and effective clinical use of these agents.
References
Alwan S, Polifka JE, Friedman JM. Angiotensin II receptor antagonist treatment during pregnancy. Birth Defects Res A Clin Mol Teratol. 2005 Feb;73(2):123-30.
Bangalore S, Kumar S, Kjeldsen SE, Makani H, Grossman E, Wetterslev J, Gupta AK, Sever PS, Gluud C, Messerli FH. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol. 2011 Jan;12(1):65-82. Epub 2010 Nov 29.
Barrios V, Calderón A, Navarro-Cid J, Lahera V, Ruilope LM. N-acetylcysteine potentiates the antihypertensive effect of ACE inhibitors in hypertensive patients. Blood Press. 2002;11(4):235-9.
Brixius K, Middeke M, Lichtenthal A, Jahn E, Schwinger RH. Nitric oxide, erectile dysfunction and beta-blocker treatment (MR NOED study): benefit of nebivolol versus metoprolol in hypertensive men. Clin Exp Pharmacol Physiol. 2007 Apr;34(4):327-31.
Burnier M. Angiotensin II type 1 receptor blockers. Circulation. 2001 Feb 13;103(6):904-12. Burnier M, Pruijm M, Wuerzner G. Treatment of essential hypertension with calcium channel blockers: what is the place of lercanidipine? Expert Opin Drug Metab Toxicol. 2009 Aug;5(8):981-7.
Chang CH, Lin JW, Wu LC, Lai MS. Angiotensin receptor blockade and risk of cancer in type 2 diabetes mellitus: a nationwide case-control study. J Clin Oncol. 2011 Aug 1;29(22):3001-7. Epub 2011 Jun 20.
Chrysant SG, Chrysant GS, Dimas B. Current and future status of beta-blockers in the treatment of hypertension. Clin Cardiol. 2008 Jun;31(6):249-52.
Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, Hall K, Ray WA.Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006 Jun 8;354(23):2443-51.
Crowley SD, Coffman TM. In hypertension, the kidney rules. Curr Hypertens Rep. 2007 Apr;9(2):148- 53.
Davis RL, Eastman D, McPhillips H, Raebel MA, Andrade SE, Smith D, Yood MU, Dublin S, Platt R. Risks of congenital malformations and perinatal events among infants exposed to calcium channel and beta-blockers during pregnancy. Pharmacoepidemiol Drug Saf. 2011 Feb;20(2):138-45. doi: 10.1002/pds.2068. Epub 2010 Nov 15.
Diav-Citrin O, Shechtman S, Halberstadt Y, Finkel-Pekarsky V, Wajnberg R, Arnon J, Di Gianantonio E, Clementi M, Ornoy A. Pregnancy outcome after in utero exposure to angiotensin converting enzyme inhibitors or angiotensin receptor blockers. Reprod Toxicol. 2011 May;31(4):540-5. Epub 2011 Feb 18.
Duarte JD, Cooper-DeHoff RM. Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics. Expert Rev Cardiovasc Ther. 2010 Jun;8(6):793-802.
Egom EE, Chirico D, Clark AL. A review of thiazide-induced hyponatraemia. Clin Med. 2011 Oct;11(5):448-51.
Elliott WJ. Drug interactions and drugs that affect blood pressure. J Clin Hypertens (Greenwich). 2006 Oct;8(10):731-7.
Fares A. Night-time exogenous melatonin administration may be a beneficial treatment for sleeping disorders in beta blocker patients. J Cardiovasc Dis Res. 2011 Jul;2(3):153-5
. Fogari R, Zoppi A. A drug safety evaluation of valsartan. Expert Opin Drug Saf. 2011 Mar;10(2):295- 303. Epub 2010 Dec 11.
Francis ME, Kusek JW, Nyberg LM, Eggers PW. The contribution of common medical conditions and drug exposures to erectile dysfunction in adult males. J Urol. 2007 Aug;178(2):591-6; discussion 596. Epub 2007 Jun 13.
Golik A, Zaidenstein R, Dishi V, Blatt A, Cohen N, Cotter G, Berman S, Weissgarten J. Effects of captopril and enalapril on zinc metabolism in hypertensive patients. J Am Coll Nutr. 1998 Feb;17(1):75-8.
Gorre F, Vandekerckhove H.Beta-blockers: focus on mechanism of action. Which beta-blocker, when and why? Acta Cardiol. 2010 Oct;65(5):565-70.
Hansen PB, Poulsen CB, Walter S, Marcussen N, Cribbs LL, Skøtt O, Jensen BL. Functional importance of L- and P/Q-type voltage-gated calcium channels in human renal vasculature. Hypertension. 2011 Sep;58(3):464-70. Epub 2011 Jul 25.
Hayduk K, Kraul H. Efficacy and safety of spirapril in mild-to-moderate hypertension. J Cardiovasc Pharmacol. 1999 Aug;34 Suppl 1:S19-23.
Hines LE, Murphy JE. Potentially harmful drug-drug interactions in the elderly: a review. Am J Geriatr Pharmacother. 2011 Dec;9(6):364-77. Epub 2011 Nov 11. Izzo JL Jr, Weir MR. Angiotensin-converting enzyme inhibitors. J Clin Hypertens (Greenwich). 2011 Sep;13(9):667-75. Epub 2011 Jul 18.
Kaplan NM. The calcium channel blocker controversy. Hypertens Res. 1996 Jun;19(2):57-64. Kendall MJ. Clinical relevance of pharmacokinetic differences between beta blockers. Am J Cardiol. 1997 Nov 13;80(9B):15J-19J.
Kjeldsen K. Hypokalemia and sudden cardiac death. Exp Clin Cardiol. 2010 Winter;15(4):e96-9.
Ko DT, Hebert PR, Coffey CS, Sedrakyan A, Curtis JP, Krumholz HM. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA. 2002 Jul 17;288(3):351-7.
Koizumi K, Ito S. [Hypertension complicated with chronic kidney disease]. Nihon Rinsho. 2011 Nov;69(11):2015-9. [Article in Japanese]
Koren-Michowitz M, Dishy V, Zaidenstein R, Yona O, Berman S, Weissgarten J, Golik A. The effect of losartan and losartan/hydrochlorothiazide fixed-combination on magnesium, zinc, and nitric oxide metabolism in hypertensive patients: a prospective open-label study. Am J Hypertens. 2005 Mar;18(3):358-63.
Koziróg M, Poliwczak AR, Duchnowicz P, Koter-Michalak M, Sikora J, Broncel M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J Pineal Res. 2011 Apr;50(3):261-6. doi: 10.1111/j.1600-079X.2010.00835.x. Epub 2010 Dec 8.
Krämer BK, Endemann D. [Cardiac risks of hypokalemia and hypomagnesemia]. Ther Umsch. 2000 Jun;57(6):398-9. [Article in German]
Leone A, Landini L, Leone A. Epidemiology and Costs of Hypertension-related Disorders. Curr Pharm Des. 2011;17(28):2955-72.
Luijendijk HJ, van den Berg JF, Hofman A, Tiemeier H, Stricker BH. Beta-blockers and the risk of incident depression in the elderly. J Clin Psychopharmacol. 2011 Feb;31(1):45-50.
Makarounas-Kirchmann K, Glover-Koudounas S, Ferrari P. Results of a meta-analysis comparing the tolerability of lercanidipine and other dihydropyridine calcium channel blockers. Clin Ther. 2009 Aug;31(8):1652-63.
Mas S, Gassò P, Alvarez S, Ortiz J, Sotoca JM, Francino A, Carne X, Lafuente A. Pharmacogenetic predictors of angiotensin-converting enzyme inhibitor-induced cough: the role of ACE, ABO, and BDKRB2 genes. Pharmacogenet Genomics. 2011 Sep;21(9):531-8.
Matchar DB, McCrory DC, Orlando LA, Patel MR, Patel UD, Patwardhan MB, Powers B, Samsa GP, Gray RN. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med. 2008 Jan 1;148(1):16-29. Epub 2007 Nov 5.
Misiewicz A, Jelen B, Dziewit T, Radwan K, Srodon-Sikora I. [Levels of copper, zinc and vitamin C in erythrocytes of humans taking nifedipine]. Pol Arch Med Wewn. 1998 May;99(5):398-402. [Article in Polish]
Nayler WG, Dillon JS. Calcium antagonists and their mode of action: an historical overview. Br J Clin Pharmacol. 1986;21 Suppl 2:97S-107S.
Noll G, Lüscher TF. Comparative pharmacological properties among calcium channel blockers: T-channel versus L-channel blockade. Cardiology. 1998;89 Suppl 1:10-5.
Ott SM, LaCroix AZ, Ichikawa LE, Scholes D, Barlow WE. Effect of low-dose thiazide diuretics on plasma lipids: results from a double-blind, randomized clinical trial in older men and women. J Am Geriatr Soc. 2003 Mar;51(3):340-7.
Patten SB. Propranolol and depression: evidence from the antihypertensive trials. Can J Psychiatry. 1990 Apr;35(3):257-9.
Parish RC, Miller LJ. Adverse effects of angiotensin converting enzyme (ACE) inhibitors. An update. Drug Saf. 1992 Jan-Feb;7(1):14-31.
Piepho RW. Overview of the angiotensin-converting-enzyme inhibitors. Am J Health Syst Pharm. 2000 Oct 1;57 Suppl 1:S3-7.
Rabi DM, Daskalopoulou SS, Padwal RS et al. The 2011 Canadian Hypertension Education Program recommendations for the management of hypertension: blood pressure measurement, diagnosis, assessment of risk, and therapy. Can J Cardiol. 2011 Jul-Aug;27(4):415-433.e1-2.
Raebel MA. Hyperkalemia Associated with Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers. Cardiovasc Ther. 2011 Jan 26. doi: 10.1111/j.1755- 5922.2010.00258.x. [Epub ahead of print]
Ram CV. Beta-blockers in hypertension. Am J Cardiol. 2010 Dec 15;106(12):1819-25. Epub 2010 Nov 2.
Richard S. Vascular effects of calcium channel antagonists: new evidence. Drugs. 2005;65 Suppl 2:1-10.
Robitaille C, Dai S, Waters C, Loukine L, Bancej C, Quach S, Ellison J, Campbell N, Tu K, Reimer K, Walker R, Smith M, Blais C, Quan H. Diagnosed hypertension in Canada: incidence, prevalence and associated mortality. CMAJ. 2011 Nov 21. [Epub ahead of print]
Salort-Llorca C, Mínguez-Serra MP, Silvestre-Donat FJ. Interactions between ibuprofen and antihypertensive drugs: incidence and clinical relevance in dental practice. Med Oral Patol Oral Cir Bucal. 2008 Nov 1;13(11):E717-21.
Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005 Oct 19;(4):CD003566.
Sarafidis PA, Georgianos PI, Lasaridis AN. Diuretics in clinical practice. Part I: mechanisms of action, pharmacological effects and clinical indications of diuretic compounds. Expert Opin Drug Saf. 2010 Mar;9(2):243-57.
Semple PF. Putative mechanisms of cough after treatment with angiotensin converting enzyme inhibitors. J Hypertens Suppl. 1995 Sep;13(3):S17-21.
Sica DA, Carter B, Cushman W, Hamm L. Thiazide and loop diuretics. J Clin Hypertens (Greenwich). 2011 Sep;13(9):639-43. Epub 2011 Jul 27.
Sica DA. Angiotensin receptor blockers: new considerations in their mechanism of action. J Clin Hypertens (Greenwich). 2006 May;8(5):381-5. Review.
Sica DA. Interaction of grapefruit juice and calcium channel blockers. Am J Hypertens. 2006a Jul;19(7):768-73.
Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol. 2010 Jul;11(7):627-36. Epub 2010 Jun 11.
Stoschitzky K, Sakotnik A, Lercher P, Zweiker R, Maier R, Liebmann P, Lindner W. Influence of beta-blockers on melatonin release. Eur J Clin Pharmacol. 1999 Apr;55(2):111-5.
Takaichi K, Takemoto F, Ubara Y, Mori Y. Analysis of factors causing hyperkalemia. Intern Med. 2007;46(12):823-9. Epub 2007 Jun 15.
Taylor AL, Wright JT Jr. Should ethnicity serve as the basis for clinical trial design? Importance of race/ethnicity in clinical trials: lessons from the African-American Heart Failure Trial (A-HeFT), the African-American Study of Kidney Disease and Hypertension (AASK), and the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Circulation. 2005 Dec 6;112(23):3654-60; discussion 3666.
Thornton SN. Thirst and hydration: physiology and consequences of dysfunction. Physiol Behav. 2010 Apr 26;100(1):15-21. Epub 2010 Mar 6.
van der Woude HJ, Zaagsma J, Postma DS, Winter TH, van Hulst M, Aalbers R. Detrimental effects of beta-blockers in COPD: a concern for nonselective beta-blockers. Chest. 2005 Mar;127(3):818-24.
Verbeek DE, van Riezen J, de Boer RA, van Melle JP, de Jonge P. A review on the putative association between beta-blockers and depression. Heart Fail Clin. 2011 Jan;7(1):89-99.
Volpe M, Morganti A; Executive Committee of the Italian Society of Hypertension. 2010 position paper of the Italian Society of Hypertension (SIIA): angiotensin receptor blockers and risk of cancer. High Blood Press Cardiovasc Prev. 2011 Mar 1;18(1):37-40.
Wang MT, Su CY, Chan AL, Lian PW, Leu HB, Hsu YJ. Risk of digoxin intoxication in heart failure patients exposed to digoxin-diuretic interactions: a population-based study. Br J Clin Pharmacol. 2010 Aug;70(2):258-67.
Westphal S, Rading A, Luley C, Dierkes J. Antihypertensive treatment and homocysteine concentrations. Metabolism. 2003 Mar;52(3):261-3.
Wiysonge CS, Bradley H, Mayosi BM, Maroney R, Mbewu A, Opie LH, Volmink J. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2007 Jan 24;(1):CD002003.
Wright JM, Musini VM. First-line drugs for hypertension. Cochrane Database Syst Rev. 2009 Jul 8;(3):CD001841.
Yoon C, Yang HS, Jeon I, Chang Y, Park SM. Use of angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers and cancer risk: a meta-analysis of observational studies. CMAJ. 2011 Oct 4;183(14):E1073-84. Epub 2011 Aug 29.