Chronic Venous Insuffi ciency (CVI)
Integrative management approaches
Abstract
Chronic venous insuffi ciency (CVI), also known as varicose vein disease, is seen with comorbid conditions of ischemic heart disease, type II diabetes, and obesity which are growing in the Western world and as such the management and support of CVI is a necessity. Hallmark infl ammation and edema in the extremities in CVI is moderately well-managed to date with compression stockings and diuretics in conventional therapy. Recent exploration of the role of reactive oxygen species at the cellular level elucidates more causal reasons for this infl ammation and edema. Antioxidant therapies, protective nitric oxide supplied through acupuncture, dietary modifi cation in addition to herbal supports such as diosmin, hespiridin, and horse chestnut provide exciting future avenues for research of the maintenance and slowing of tissue damage in individuals with CVI.
Varicose vein disease, or chronic venous insuffi ciency (CVI), is a common health condition, aff ecting up to 65% of women and 50% of men over the age of 45, and accompanied by comorbidities of cardiovascular disease, type II diabetes, and obesity (Bergan 2006, Burkett 1973, McLaff erty 2008, Robertson 2008). Collapsed venous valves lead to pooled venous fl uid in tissues, inducing infl ammation that progresses to signifi cant tissue damage. To date, the etiology of this process has not been well elucidated. In recent years, however, the endothelial dysfunction of CVI has been viewed as related not only to the eff ects of long-term infl ammation but also to oxidative stress and a decreased capacity for antioxidant defence (Carrasco 2009). More rigorous identifi cation of herbal anti-infl ammatory supports for vascular dysfunction as well as the use of antioxidant-enhancing mechanisms such as acupuncture and L-arginine has made the role of complementary supports for CVI a more mainstream option for treatment in coming years.
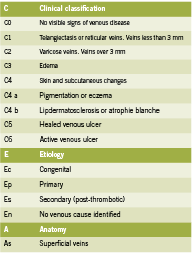
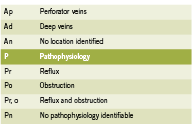
Attempts to properly organize CVI symptomatology lead to the development of the CEAP rubric in 1995. Since tested for its consistency, the CEAP acronym organizes CVI by clinical (C), etiological (E), anatomical (A) and physiological (P) condition states (Porter 1995) and has been proven to be the best objective manner of testing and classifying CVI between patients and clinicians (Kistner 1996). Table 1 outlines the classifi cations for CEAP.
Common conventional treatments include compression therapy and sclerotherapy, with compression therapy the current gold standard (Braunwald 2001). Compression therapy (including specialized stockings; “TEDS”) provides mechanical support to minimize fl uid pooling and maximize limb function in patients with progressed disease.
e progression of CVI is characterized by infl ammatory mediators that cause collagen & elastin remodelling in the endothelium, and this results in loss of vessel wall integrity. is remodelling, coupled with ongoing infl ammation and reactive oxygen species production leads to vein collapse (Raff etto 2008). Vasorelaxation responses that are controlled by norepinephrine, acetylcholine, and serotonin dynamics are all decreased in individuals with CVI (Belcaro 1989, Belcaro 1988, Georgescu 2009).
Reactive oxygen species (ROS) play a key role in mediating chronic infl ammation; ROS cause oxidation of lipid membranes (Guzik 2011), and DNA damage that further amplifi es the infl ammatory response (Halliwell 2000). ROS are reactive chemical forms of oxygen that include superoxide anions (O2-) and hydroxyl radicals that are capable of damaging cells. Antioxidant mechanisms like the enzyme superoxide dismutase can minimize this damage by producing hydrogen peroxide (H2O2) from the superoxide anion (O2-) which will then be converted by catalase to water. However, in enzymatic defi ciency and infl ammation, superoxide anions can go “unchecked” by normal antioxidant mechanisms. Additionally, the enzymes NAD(P)H oxidase, cyclooxygenase, nitric oxide synthase, and xanthine oxidase can all form superoxide radicals (O2-) from molecular oxygen (O2), enhancing the problem (Halliwell 2000, Klaubunde 2012, Krzysciak 2011).
Produced by L-arginine reacting with nitric oxide synthase, NO is protective to the endothelium (Meletis 2006). Increased NO and its metabolites cause an increase in guanylate cyclase (GC) and cGMP, which leads to relaxation of vascular smooth muscle and vasodilation (Ignarro 1990, Ignarro 1993, Klaubunde 2012, Meletis 2006). NO is also anti-infl ammatory and anti-thrombotic as it discourages platelet aggregation in the endothelium and pro-inflammatory mediators. The presence of superoxide anion scavenges NO, reducing its bioavailability leading to vasoconstriction, increased leukocyte-endothelial adhesion through intracellular adhesion molecules (ICAM), and structural blood vessel changes contributing to vein collapse. Indeed, decreased nitric oxide (NO) is seen in atherosclerosis, type II diabetes, hypertension, and smoking (Guzik 2002). Inflammation causes more fluid to be drawn into the collapsed venous systems, exacerbating the condition (Krzyściak 2011, Yasim 2008). Hence, antioxidant therapy to support NO production is beneficial for CVI, as is management of inflammation to decrease immunemediated DNA destruction.
Integrative Treatments for Oxidative Stress with CVI
Acupuncture is a therapy that offers a multitude of beneficial effects for CVI. Acupuncture has proven to have analgesic effects via stimulation of alpha, delta and C-fibres in the skin and muscles responsible for pain perception; these fibres initiate cascades inducing peripheral vasodilation and production of proinflammatory agents such as substance P, opioids, somatostatin, and vasoactive intestinal peptide. As a result, acupuncture has the ability to influence fluid movement and edema in tissues (Cassileth 2011, Lewith 1983, Ma 2003, Sprott 1998). It is believed that acupuncture meridians and points themselves are regions of low electrical resistance and high electrical conductivity in the nervous system, and as a result may be able to impact the sympathetic nervous system, the release of substance P, and decreased pain perception (Ahu 1981, Ma 1993, Zhu 1989).
An increased presence of NO has been found in skin areas where acupuncture meridians are found, as well as generally increased NO levels in the skin following acupuncture in vitro and in vivo in both human and animal models (Li 2003, Ma 2003). Twenty subjects with hand and forearm pain were exposed to two minutes of either acupuncture at the points LI 4, P6, P8, L6, and H5 or non-invasive sham acupuncture for the same length of time. Non-sham acupuncture sessions were found to have significantly increased blood flow around the chosen points both five and 60 minutes following the session, as well as increasing blood NO concentration (Tchiyua 2007). In other studies, however, sham acupuncture has also been found to have blood flow increasing effects, and further studies will be needed to understand the NO-enhancing capacity of standard acupuncture to truly distinguish between placebo effects (Lewith 1983, Park 2011, Takakura 2011,). A case report exists of a 69-year old male with bilateral venous ulcers and rheumatoid arthritis who was treated with compression bandages and an anti-inflammatory regime (NSAIDS, sulfasalazine, methotrexate), but without adequate healing; when treated with three weekly sessions of acupuncture at points ST36, KI3 and LR3 bilaterally, full healing of the ulcer on the left leg was seen within three weeks (Mears 2003). Since compression therapy is the current most utilized treatment for CVI, a larger study is being conducted to compare acupuncture with compression bandages (Vas 2011).
Botanical Agents
Horse chestnut seed extract (HCSE) has been used widely in Europe for many years for CVI, hemorrhoids, and post-operative edema, and is slowly being recognized for these purposes in North America (Tiffany 2002, Sirtori 2001). Aescins, a combination of triterpene saponins from horsechestnut, are believed to be responsible for reducing the small vessel permeability of the venous system (Ulbricht 2002). Horsechestnut also contains anti-inflammatory bioflavonoids (quercitin and kaempferol), proanthocyanidin A2, and venotonic coumarins (fraxin and aesculin) (Tiffany 2002, Lorenz 1960, Mrwa 1986). HCSE’s are typically standardized to contain 16-20 percent aescin, and the oral dosage is typically 100-150 mg aescin/day in divided doses as a dried seed or 2-3 mL daily as a tincture (Abascal 2007, Tiffany 2002). HSCE cannot be used in those with kidney disease or broken skin due to irritating effects (Abascal 2007).
On a molecular level, HCSE is thought to act by increasing sensitization to calcium ions, thus decreasing permeability of small vessels and enhancing venous contractile activity which decreases edema (Tiffany 2002). Its immune-stabilizing properties include the ability to decrease leukocyte migration and adherence, and reduce local levels of inflammatory mediators (Pangieri 1992). Systematic reviews of RCTs investigating HCSE have been conducted, with the most recent in 2006 (Pittler 2004, Pittler 2006). Pittler concluded that HCSE treatment was associated with a significant improvement in CVI symptoms of leg pain, itching, and fatigue, as well as reduction in lower-leg volume as a short-term treatment (2006). RCTs have also compared HCSE with compression therapy. Ottillinger and Greeske found that both therapies were equally effective in earlier stages of CVI, when irreparable damage has not occurred in the venous system; however in later stages of CVI, only compression therapy was effective compared to placebo (p <0.001), whereas HCSE was not (p = 0.115) (Diehm 1996, Ottillinger 2001). Further research needs to be done to determine the clinical stages in which HSCE is best administered.
Diosmin and hespiridin are bioflavonoids used for CVI, and have been patented as Daflon ® or a micronized purified flavonoid fraction (MPFF). Danielsson (2002) compared 101 patients randomised to either MPFF twice daily (Daflon ® 500 mg, standardized as 90% diosmin and 10% flavonoids expressed as hesperidin) or placebo for 60 days. Evaluation was with CEAP (subjective symptoms of pain, edema, fatigue rated from 1-3 by patients), plethysmography (foot-volumetry), and duplexultrasonography before and after the treatments. Night cramps were significantly improved (p=0.03) with MPFF compared with placebo; however there was no significant effect on tiredness, heaviness, pain, and swelling as well as duplex-ultrasonography compared to placebo (Danielsson 2002).
Pycnogenol, a French maritime pine bark extract was associated with endothelial improvements in CVI (Fitzpatrick 1998) when 150 mg or 300 mg daily were compared with Daflon® at 1000 mg/day for eight weeks in a randomized trial (Cesarone 1996). Pycnogenol resulted in significant (p<0.05) improvements in rate of edema formation, resting ankle flux, and rate of ankle swelling, compared to Daflon treatment at both the lower dose and in the higher dose groups (Cesarone 2006). Limitations of these studies include lack of standardization of diagnostic criteria, and use of subjective scoring as opposed to standardized CEAP scoring (Martinez-Zapata 2008). The diagnostic methods for the comparison of blood flow in the extremities are also varied (Martinez-Zapata 2008). Comparatively, pycnogenol demonstrates higher improvements in endothelial CVI attributes (edema and its rate) compared with the more popular diosmin and hespiridin to date.
Obesity, Diet and Chronic Venous Insufficiency
A plenitude of research supports physical activity and weight management in the improvement of CVI (Brand 1988). Malonylodialdehyde (MDA), a by-product of lipid peroxidation created by oxidative stress was examined in a group diagnosed with 2nd or 3rd degree CVI (n=62) compared to controls. BMI was assessed as a covariable. Not surprisingly, MDA was higher in patients with cardiovascular disease compared with the control group (p < or = 0.0005), however, MDA concentration was also significantly higher in obese patients compared to those with a normal BMI (p < or = 0.001) (Kózka 2009). These findings bring further attention to the use of lifestyle and dietary modifications as a way to reduce markers of inflammation, impact NO concentration, and benefit CVI as well as CVD risk. Some nutritional agents that may also assist in this include: folic acid and its substrates, L-arginine, and non-genetically modified soy. L-arginine is an important substrate for NO production, while folic acid and its cofactors vitamin B6 and B12 reduce homocysteine levels that contribute to oxidative destruction of NO (Meletis 2006). Folate inhibits intracellular superoxide production, which increases the half-life of NO, allowing for greater vasodilation and further tissue protection (Meletis 2006). A diet emphasizing increased healthy fats, complex carbohydrates, and plant-based antioxidants as per the Mediterranean diet are beneficial in cardiovascular disease as well as CVI.
Future Directions for CVI: Influence of Estrogen
Women have a twofold higher incidence of CVI compared with men (Brand 1988). Serum levels of estradiol have been significantly associated with varicose veins, especially in post-menopausal women (Ciardullo 2000). Estrogen has been shown to upregulate vascular relaxation in the endothelium via nitric oxide (NO) and prostacyclin (PGI2) (Orsall 2004). Animal studies comparing the endothelium of male and female rats (n=12) have revealed significant sex differences in vasoconstriction/ relaxation, such as enhanced endothelium-dependent vasodilation and increased estrogen receptor activity in female rats, even independent of a NO pathway, suggesting there is an additional influence of estrogen on vasodilation, capable of acting synergistically with vasodilation effects of NO (Raffetto 2010). The influences of sex-hormone binding globulin (SHBG) and testosterone were also tested in the study, but were not found to influence varicose vein activity. Whether these findings can be replicated in human studies remains to be seen.
CVI is an early warning sign of cardiovascular disease. Reactive oxidative species are found in higher concentration in varicose tissues, and these respond well to interventions that reduce oxidative stress and enhance nitric oxide presence in kind, including acupuncture, horse chestnut seed extract, hespiridin and diosmin and an emphasis on healthy dietary habits. Comparing these interventions with compression therapy and better developing our understanding of the role of estrogen will lead to more effective treatment of CVI.
References
Abascal K and Yarnell E. Botanicals for Chronic Venous Insufficiency. Alt Complement Ther. 2007; 304-312.
Ahu ZX 1981. Research advances in the electrical specificity of meridians and acupuncture points. Am J Acup. 1981; 9:203-213.
Belcaro G. The role of transcutaneous PCO2 measurements in association with laser Doppler flowmetry in venous hypertension. Phlebology. 1988;3:189.
Belcaro G, Grigg M, Rulo A, Nicolaides AN. Blood flow in the perimalleolar skin in relation to posture in patients with venous hypertension. Ann Vasc Surg. 1989;1:5.
Bergan JJ, Schmid-Schonbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006 Aug 3; 355(5): 488-96.
Brand FN, Dannenberg AL, Abbott RD, Kannel WB. The epidemiology of varicose veins: the Framingham Study. Am J Prev Med. 1988; 4(2): 96-101.
Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL. Harrison’s Principles of Internal Medicine. 15th Edition. McGraw-Hill Professional, 2001. Burkitt DP. Some Diseases Characteristic of Modern Western Civilization; Occasional Review. British Medical Journal. 1973; 1: 274-278.
Carrasco OF, Ranero A, Hong E, Vidrio H. Endothelial function impairment in chronic venous insufficiency: effect of some cardiovascular protectant agents. Angiology. 2009; 60: 763-771.
Cassileth BR, Van Zee KJ, Chan Y, Coleton MI, Hudis CA, Cohen S, Lozada J, Vickers AJ. A safety and efficacy pilot study of acupuncture for the treatment of chronic lymphoedema. Acupunct Med. 2011;29:170–172.
Ciardullo AV, Panico S, Bellati C, Rubba P, Rinaldi S, Iannuzzi A, Cioffi V, Iannuzzo G, Berrino F. High endogenous estradiol is associated with increased venous distensibility and clinical evidence of varicose veins in menopausal women. J Vasc Surg. 2000 Sep; 32(3): 544-9.
Cesarone MR, Belcaro G, Rohdewald P, Pellegrini L, Ledda A, Vinciguerra G, Ricci A, Gizzi G, Ippolito E, Fano F, Dugall M, Acerbi G, Cacchio M, DiRenzo A, Hosoi M, Stuard S, and Corsi M. Comparison of Pycnogenol® and Daflon® in Treating Chronic Venous Insufficiency: A Prospective, Controlled Study. Clin Appl Thromb Hemost. 2006; 12: 205.
Danielsson G, Jungbeck C, Peterson K, and Norgren L. A Randomised Controlled Trial of Micronized Purified Flavonoid Fraction vs. Placebo in Patients with Chronic Venous Disease. Eur J Vasc Endovasc Surg. 2002; 23: 73-76.
Diehm C, Trampisch HJ, Lange S, Schmidt C. Comparison of leg compression stocking and oral horse-chestnut seed extract therapy in patients with chronic venous insufficiency. Lancet. 1996;347(8997):292-294.
Fitzpatrick DF, Bing B, Rohdewald P. Endothelium-dependent vascular effects of Pycnogenol. J Cardiovasc Pharmacol. 1998;32:509–515.
Halliwell B, and Gutteridge JMC. 2000. Free radicals in biology and medicine. 3rd edn. Oxford University Press.
Horse chestnut (Aesculus hippocastanum L.). http:// www.mayoclinic.com/health/ horse-chestnut/NS_ patient-horsechestnut [Accessed December 2 2011]
Georgescu A, Alexandru N, Popov D, Amuzescu M, Andrei E, Zamfir C, Maniu H, Badila A. Chronic venous insufficiency is associated with elevated level of circulating microparticles. J Thromb Haemost. 2009; 7: 1566–75.
Guzik TJ, Mussa S, Gastaldi D, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: Role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002; 105: 1656-1662.
Guzik B, Chwala M, Matuski P, Ludew D, Skiba D, Wilk G, Mrowiecki W, Batko B, Cencora A, Kapelak B, Sadowski J, Korbut R, Guzik TJ . Mechanisms of increased vascular superoxide production in human varicose veins. Archiwum Medycyny Wewnetrzej (Polish). 2011; 121 (9) 279-284.
Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990; 30:535–560.
Ignarro LJ, Fukuto JM, Griscavage JM, Rogers NE, Byrns RE. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate. Comparison with enzymatically formed nitric oxide from L-arginine. Proc Natl Acad Sci. 1993;90:8103–8107.
Kistner RL, Eklof B, and Masuda EM. Diagnosis of Chronic Venous Disease of the Lower Extremities: the “CEAP” Classification. Mayo Clin Proc. 1996; 71:338-345
Klaubunde, CM. Cardiovascular Physiology Concepts. Reactive Oxygen Species. Accessed April 2012 http://www.cvphysiology.com/Blood%20Flow/BF016.htm
Klaubunde CM. Cardiovascular Physiology Concepts. Nitric Oxide. Accessed April 2012. http://www.cvphysiology.com/Blood%20Flow/BF011.htm
Kózka M, Krzyściak W, Pietrzycka A, Stepniewski M. Obesity and its’ influence on reactive oxygen species (ROS) in the blood of patients with varicose veins of the lower limbs [Article in Polish]. Przegl Lek. 2009; (66): 213-7.
Krzyściak W and Kózka M. Generation of reactive oxygen species by a sufficient, insufficient and varicose vein wall. Acta Biochemica Polonica. 2011; 58(1): 89–94
Lewith GT, Field J, Machin D. Acupuncture compared with placebo in post-herpetic pain. Pain. 1983;17:361–8
Li S, Chen K, Wu Y, Jiao J, Tao L. Effects of warm needling at zusanli (ST 36) on NO and IL-2 levels in the middle-aged and old people. J Tradit Chin Med. 2003; 23:127-128.
Lorenz D, Marek ML. The active therapeutic principle of horse chestnut (Aesculus hippocastanum). Part 1. Classification of the active substance. Arzneimittelforschung. 1960;10:263-272. [Article in German]
Ma SX. Enhanced nitric oxide concentrations and expression of nitric oxide synthase in acupuncture points/meridians. J Altern Complement Med. 2003; 9:207-215.
Martinez-Zapata MJ, Bonfill Cosp X, Moreno RM, Vargas E, Capella D.
Phlebotonics for venous insufficiency (Review). The Cochrane Review 2008, Issue 4. Mears, Tim. Acupuncture for chronic venous ulcerations [Case report]. Acupuncture in Medicine. 2003; 21: 150-152.
McLafferty RB, Passman MA, Caprini JA, Rooke TW, Markwell SA, Lohr JM, Meissner MH, Eklöf BG, Wakefield TW, Dalsing MC. Increasing awareness about venous disease: The American Venous Forum expands the National Venous Screening Program. J Vasc Surg. 2008 Aug;48(2):394-9.
Meletis CD and Zabriskie N. Nitric Oxide, a Powerful Clinical Therapy. Alt Comp Therapies. 2006.
Mrwa U, Guth K, Haist C, et al. Calcium-requirement for activation of skinned vascular smooth muscle from spontaneously hypertensive (SHRSP) and normotensive control rats. Life Sci. 1986;38:191-196.
Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–R249.
Ottillinger B and Greeske K. Rational therapy of chronic venous insufficiency-chances and limits of the therapeutic use of horse-chesnut seeds extract. BMC Cardiovascular Disorders. 2001; 1:5.
Panigati D. The pharmacology of escin, a saponin from Aesculus hippocastanum L. II. Pharmacodynamics of escin. Chapter I. Boll Chim Farm. 1992;131:242-246. [Article in Italian]
Park JJ, Bang H. Methodological advances needed in analysis and interpretation of sham acupuncture validation studies. Acupunct Med. 2011 Sep; 29(3): 168-9.
Pittler MH and Ernst E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Systematic Review. 2004: (2): CD003230.
Pittler MH and Ernst E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Systematic Review. 2006 Jan 25;(1):CD003230.
Porter JM, Moneta GL. Reporting standards in venous disease: an update. International Consensus Committee on Chronic Venous Disease. Journal of Vascular Surgery. 1995; 21(4):635–45.
Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008; 75: 346-359.
Raffetto JD, Qiao X, Beauregard KG, Khalil RA. Estrogen receptor-mediated enhancement of venous relaxation in female rat: implications in sex-related differences in varicose veins. J Vasc Surg. 2010 Apr; 51 (4): 972-81.
Robertson L, Evans C, Fowkes FG. Epidemiology of chronic venous disease. Phlebology. 2008; 23: 103-111.
Sirtori CR. Aescin: pharmacology, pharmacokinetics and therapeutic profile. Pharmacol Res. 2001;44:183-193.
Sprott H, Franke S, Kluge H, Hein G. Pain treatment of fibromyalgia by acupuncture. Rheumatol Int. 1998; 18(1): 35-6.
Takakura N, Takayama M, Kawase A, Yajima H. Double blinding with a new placebo needle: a validation study on participant blinding. Acupunct Med. 2011 Sep;29(3):203-7.
Tiffany, N et al. Horsechestnut: A multidisciplinary clinical review [from Natural Standard]. Journal of Herbal Pharmacotherapy. 2002; 2(1).
Tsuchiya M, Sato EF, Inoue M, Asada A. Acupuncture enhances generation of nitric oxide and increases local circulation. Anesth Analg. 2007;104:301-307.
Vas J, Modesto M, Mendez C, Perea-Milla E, Aguilar I, Carrasco-Lozano JM, Fasu V, and Martos F. Effectiveness of acupuncture, special dressings and simple, low-adhererence dressings for healing venous leg ulcers in primary healthcare: study protocol for a cluster-randomized open-labeled trial. BMC Complementary and Alternative Medicine. 2008; 8:29; 1472-6882.
Vein Clinics of America. Chronic venous insufficiency. http://www.veinclinics.com/ cme/skin-findings. html#ulcer. [Accessed December 9, 2011]
Yasim A, Kilinc M, Aral M, Oksuz H, Kabalci M, Eroglu E, Imrek. Serum concentration of procoagulant, endothelial and oxidative stress markers in early primary varicose veins.Phlebology/Venous Forum of the Royal Society of Medicine. 2008;23: 15–20.