A cetyl-L-Carnitine
Clinical application as a neuroprotective agent
Introduction
As an essential regulator of mitochondrial function and fatty acid metabolism, L-carnitine is relatively well known for its ability to improve muscle function in conditions such as cardiac ischemia, and is often used in the treatment of other muscular conditions such as fibromyalgia. The ability of carnitine to impact neurological function, however, is perhaps less well known. Surprisingly, there is a fairly well developed corpus around the use of acetyl-L-carnitine (ALC) in patients with neurological conditions ranging from age related dementia, substance withdrawal, depression, Down syndrome, attention deficient hyperactivity disorder, neuropathy, and encephalopathy secondary to liver failure (Arnold 2007, Janiri 2009, Malaguarnera 2008, Montgomery 2003, Pueschel 2006, Youle 2007, Zanardi 2006). This paper reviews the neuroprotective effects of ALC, with a narrowed focus on its effects in Alzheimer’s disease (AD), substance withdrawal, and toxic peripheral neuropathies.
Physiology
As reviewed previously, carnitine is a key factor in the transport of fatty acids between the cytoplasm and the mitochondria, facilitating production of ATP, and appears to be in especially high demand under ischemic conditions. Acetyl-L-carnitine passes the blood-brain barrier through an active transport process and is highly concentrated in the brain, especially in the hypothalamus (Thal 2000, Virmani 2004), and is therefore the main focus of carnitine research in the area of neurological disease.
ALC exerts its neuroprotective effect through a variety of mechanisms. According to Quatraro, ALC has analgesic effects by acutely increasing blood levels of the endogenous opioid peptide beta-endorphin, neurotropic effects by upregulating nerve growth factor receptors on the brain and preventing accumulation of lipofuscin, and metabolic effects by increasing the oxidative metabolism of neurons (Calvani 1992, Quatraro 1995). Pettegrew used magnetic resonance spectroscopy to show that administration of ALC normalized brain levels of high energy phosphates in patients with AD compared to healthy controls (1995). ALC also has antioxidant effects, increasing glutathione and decreasing malondialdehyde (Thal 2000). Finally, acetyl-L-carnitine is thought to have cholinergic effects through facilitation of intracellular acetylcholine synthesis; carnitine transports acetyl groups out of the mitochondra into the cytoplasm where they can be used for ACh production (Thal 2000, Virmani 2004).
Clinical Evidence: Cognitive Impairment and/ or Alzheieimer’s DiDisease
ALC has been shown to improve cognitive function in elderly patients without dementia. Two randomized double blind placebo controlled trials showed ALC to improve mental fatigue in patients over 70 and over 100 years of age, respectively, along with concomitant improvements in physical function (Malaguarnera 2008, 2007). A third trial reported significant improvements in cognitive function, memory, emotional affect, and mood when evaluated in 481 elderly patients without AD (Salvioli 1994).
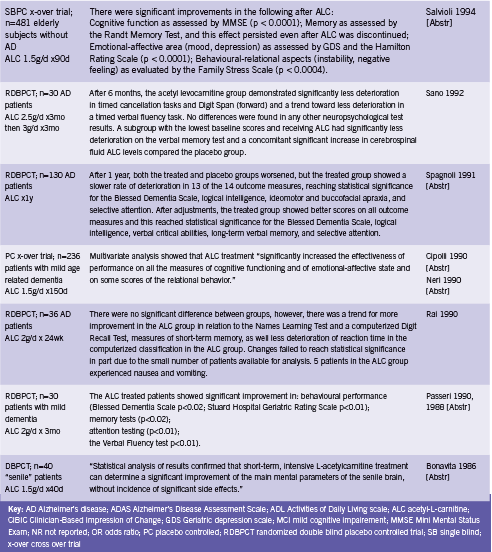
Two meta analyses have evaluated the effect of ALC for use in AD and/ or mild cognitive impairment (MCI) (Hudson 2003, Montgomery 2003). Montgomery included 21 randomized double blind placebo controlled trials assessing ALC for AD or MCI and concluded that ACL should be considered for the treatment of these conditions (2003). Doses ranged from 1.5 to 3.0 g/d for between 3 to 12 months. A combined effect size was calculated that incorporated both clinical and psychometric outcomes from the trials. There was significant benefit from treatment with ALC compared to placebo, effect size ES 0.201 (95% CI 0.107-0.295), and there was also a significant effect in the pooled Clinical Global Impression of Change, ES 0.32 (95% CI 0.18-0.47). ALC was well tolerated (Montgomery 2003).
The Cochrane review updated in 2008 included 16 randomized double blind placebo controlled trials (Hudson 2003). By contrast, this analysis found that while there was a statistically significant treatment effect in favour of ALC on the Clinical Global Impression at 12 and 24 wk (OR 1.90, 95% CI 1.31-2.76 and OR 2.33, 95% CI 1.31-4.14 respectively) this was not sustained at 52 weeks (OR 0.91, 95% CI 0.58-1.43). Similar effects were seen in pooled results for the Mini Mental State Exam (MMSE), leading authors to conclude that ALC may be of limited benefit.
This review excluded several studies that were included by Mongomery on the basis of poor reporting and/ or trial design. These trials were largely earlier trials out of Italy, so poor reporting standards may be to blame. In any case, the consequent discrepancy in included studies partially explains the divergent conclusions reached by the two studies. In addition, Mongomery utilized a unique composite effect measure that may not be directly comparable to those used by Hudson by pooling data across different assessment scales. However, it seems that the possibility of ALC benefiting at least a subset of patients with Alzheimer’s or MCI should not be ruled out. Hudson explains that the mechanisms by which ALC is absorbed and metabolized in the gut and liver “suggest that a large inter-individual variability should be expected in the general population. Studies have not taken this into account in selecting the dose of ALC, and this may be a source of error” (2003). Select trials of ALC for cognitive impairment are summarized in Table 1.

One uncontrolled trial found that the addition of ALC to the treatment of acetylcholinesterase inhibitor resistant patients with AD increased the response rate from 38% to 50% (p value not given) (Bianchetti 2003). ALC may therefore be useful for augmenting the effect of other therapies, even when it is not sufficient as a stand alone intervention.
Substance Withdrawal
A handful of trials have examined the potential effectiveness of ALC in ameliorating the symptoms of withdrawal from substances including methadone, cocaine, and alcohol. Janiri 2009 found that oral ALC 2g/d significantly decreased symptoms of methadone (opiate) withdrawal compared to placebo in 30 patients undergoing a three week detoxification program. Total symptom scores during the first five days were significantly lower in the ALC group (p<0.05), and in particular the following symptoms were improved: muscle tension and muscle spasm (p<0.05), insomnia (p<0.005), and feelings of coldness (p<0.05). Pain as assessed by the Huskisson analog scale was also “considerably lower” in the ALC group from after week one until the end of the study.
One study found no impact on symptoms of cocaine withdrawal or drug cravings (Reid 2005), while two RCTs reported significant improvements in cognitive impairment, mood, and anhedonia associated with chronic alcohol abuse in abstinent patients (Martinotti 2011, Tempesta 1990).
Peripheral Neuropathy
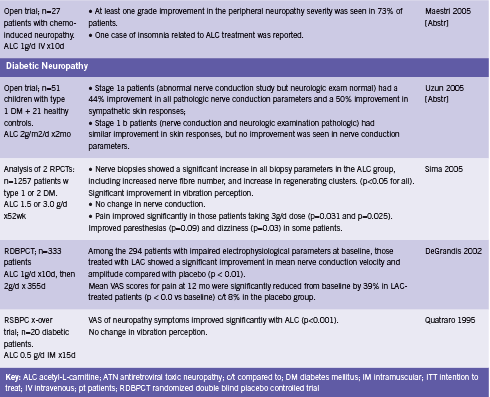
ALC appears to protect nerves from the effects of toxic drugs such as antiretroviral therapy and chemotherapy, and from the damaging effects of diabetes. One RCT and three open trials have reported improvements in pain and/ or symptom scores from administration of ALC in patients with antiretroviral toxic neuropathy (Hart 2004, Osio 2006, Scarpini 1997, Youle 2007). Two open trials have found reductions in neuropathy grade and/ or symptomatic improvements in patients with neuropathy of chemotherapy (Bianchi 2005, Maestri 2005). Four RCTs and one open trial reported improvements in pain and/ or neuropathy grade when ALC was given to patients with diabetes (De Grandis 2002, Quatraro 1995, Sima 2005). These studies are summarized in Table 2.
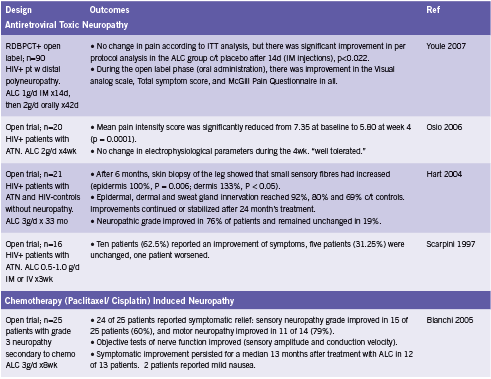
ALC has been shown to aid in the regeneration of damaged nerve tissue. Hart found that on biopsy, the skin of patients taking ALC for six months showed significant increases in the number of small sensory fibers, a 133% increase in the dermis, and 100% in the epidermis (2004). Furthermore, epidermal innervation reached up to 92% of that compared to healthy HIV- controls without neuropathy (Hart 2004). Sima demonstrated similar results in diabetic patients (2005). ALC has also been shown to improve of nerve conduction parameters, velocity and amplitude (Bianchi 2005, De Grandis 2002). Sima reported no effect on this outcome (2005), while Uzun found improvement in those patients with stage 1a diabetic neuropathy, but not those with stage 1b disease (2005).
Cerebrovascular Insufficiency
Finally, two small controlled trials have examined ALC in patients with cerebrovascular insufficiency (Arrigo 1990, Postiglione 1991). Intravenous administration of a single dose of ALC 1.5g increased cerebral blood flow compared to placebo (p<0.05) to the ischemic area but not the area corresponding to the stroke among 20 patients with a history of ischemic stroke when assessed by single proton emission computed tomography (SPECT) imaging (Postiglione 1991, Abstr). A cross over study in 12 patients undergoing rehab for cerebrovascular insufficiency reported that “significant differences between the drug [ALC] and placebo were found in memory, number and word tests and in the responses to simple stimuli and the performance of the maze test” (Arrigo 1990, Abstr).
Conclusion
Acetyl-L-carnitine possesses analgesic, neuroprotective, metabolic, and cholinergic activities. Human studies have demonstrated clinical improvements associated with ALC use in a range of neurological conditions including Alzheimer’s disease and age related cognitive impairment, psychological and cognitive effects of chronic alcohol abuse, methodone withdrawal, toxic neuropathy of various etiologies, and chronic cerebrovascular insufficiency. Although there is some question as to the consistency of its effects between individual patients especially in AD, the majority of the evidence suggests that acetyl-L-carnitine would be a valuable intervention for use in the treatment of toxic or degenerative neurological conditions where permitted by jurisdiction of practice.
Acknowledgements
The neuroprotective properties of ALC were first brought to this author’s attention by Paul Saunders ND, PhD.
References
Arnold LE, Amato A, Bozzolo H, Hollway J, Cook A, Ramadan Y, Crowl L, Zhang D, Thompson S, Testa G, Kliewer V, Wigal T, McBurnett K, Manos M. Acetyl-L-carnitine (ALC) in attention-deficit/hyperactivity disorder: a multi-site, placebo-controlled pilot trial. J Child Adolesc Psychopharmacol. 2007 Dec;17(6):791-802.
Arrigo A, Casale R, Buonocore M, Ciano C. Effects of acetyl-L-carnitine on reaction times in patients with cerebrovascular insufficiency. Int J Clin Pharmacol Res. 1990;10(1-2):133-7.
Bianchetti A, Rozzini R, Trabucchi M. Effects of acetyl-L-carnitine in Alzheimer’s disease patients unresponsive to acetylcholinesterase inhibitors. Curr Med Res Opin. 2003;19(4):350-3.
Bianchi G, Vitali G, Caraceni A, Ravaglia S, Capri G, Cundari S, Zanna C, Gianni L. Symptomatic and neurophysiological responses of paclitaxel- or cisplatin-induced neuropathy to oral acetyl-L-carnitine. Eur J Cancer. 2005 Aug;41(12):1746-50.
Bonavita E. Study of the efficacy and tolerability of L-acetylcarnitine therapy in the senile brain. Int J Clin Pharmacol Ther Toxicol. 1986 Sep;24(9):511-6.
Brooks JO 3rd, Yesavage JA, Carta A, Bravi D. Acetyl L-carnitine slows decline in younger patients with Alzheimer’s disease: a reanalysis of a double-blind, placebo-controlled study using the trilinear approach. Int Psychogeriatr. 1998 Jun;10(2):193-203.
Calvani M, Carta A, Caruso G, Benedetti N, Iannuccelli M. Action of acetyl-L-carnitine in neurodegeneration and Alzheimer’s disease. Ann N Y Acad Sci. 1992 Nov 21;663:483-6.
Cipolli C, Chiari G. [Effects of L-acetylcarnitine on mental deterioration in the aged: initial results]. Clin Ter. 1990 Mar 31;132(6 Suppl):479-510. [Abstr]
De Grandis D, Minardi C. Acetyl-L-carnitine (levacecarnine) in the treatment of diabetic neuropathy. A long-term, randomised, double-blind, placebo-controlled study. Drugs R D. 2002;3(4):223-31.
Hart AM, Wilson AD, Montovani C, Smith C, Johnson M, Terenghi G, Youle M. Acetyl-l-carnitine: a pathogenesis based treatment for HIV-associated antiretroviral toxic neuropathy. AIDS. 2004 Jul 23;18(11):1549-60.
Hudson S, Tabet N. Acetyl-L-carnitine for dementia. Cochrane Database Syst Rev. 2003;(2):CD003158. Review. PubMed PMID: 12804452.
Janiri L, Martinotti G, Tonioni F, Ghelardini C, Nicolai R, Galeotti N, Mosconi L, Calvani M, Bartolini A, Iannoni E. Acetyl-L-carnitine in the management of pain during methadone withdrawal syndrome. Clin Neuropharmacol. 2009 Jan-Feb;32(1):35-40.
Maestri A, De Pasquale Ceratti A, Cundari S, Zanna C, Cortesi E, Crinò L. A pilot study on the effect of acetyl-L-carnitine in paclitaxel- and cisplatin-induced peripheral neuropathy. Tumori. 2005 Mar-Apr;91(2):135-8.
Malaguarnera M, Cammalleri L, Gargante MP, Vacante M, Colonna V, Motta M. L-Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: a randomized and controlled clinical trial. Am J Clin Nutr. 2007 Dec;86(6):1738-44.
Malaguarnera M, Gargante MP, Cristaldi E, Vacante M, Risino C, Cammalleri L, Pennisi G, Rampello L. Acetyl-L-carnitine treatment in minimal hepatic encephalopathy. Dig Dis Sci. 2008 Nov;53(11):3018-25.
Malaguarnera M, Gargante MP, Cristaldi E, Colonna V, Messano M, Koverech A, Neri S, Vacante M, Cammalleri L, Motta M. Acetyl L-carnitine (ALC) treatment in elderly patients with fatigue. Arch Gerontol Geriatr. 2008 Mar-Apr;46(2):181-90.
Martinotti G, Andreoli S, Reina D, Di Nicola M, Ortolani I, Tedeschi D, Fanella F, Pozzi G, Iannoni E, D’Iddio S, Prof LJ. Acetyl-l-Carnitine in the treatment of anhedonia, melancholic and negative symptoms in alcohol dependent subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2011 Jan 20. [Epub ahead of print]
Montgomery SA, Thal LJ, Amrein R. Meta-analysis of double blind randomized controlled clinical trials of acetyl-L-carnitine versus placebo in the treatment of mild cognitive impairment and mild Alzheimer’s disease. Int Clin Psychopharmacol. 2003 Mar;18(2):61-71.
Neri M, Cortelloni C, De Vreese L. [Methodology of a clinical controlled study of L-acetylcarnitine]. Clin Ter. 1990 Mar 31;132(6 Suppl):457-68. Italian.
Osio M, Muscia F, Zampini L, Nascimbene C, Mailland E, Cargnel A, Mariani C. Acetyl-l-carnitine in the treatment of painful antiretroviral toxic neuropathy in human immunodeficiency virus patients: an open label study. J Peripher Nerv Syst. 2006 Mar;11(1):72-6.
Passeri M, Cucinotta D, Bonati PA, Iannuccelli M, Parnetti L, Senin U. Acetyl-L-carnitine in the treatment of mildly demented elderly patients. Int J Clin Pharmacol Res. 1990;10(1-2):75-9.
Passeri M, Iannuccelli M, Ciotti G, Bonati PA, Nolfe G, Cucinotta D. Mental impairment in aging: selection of patients, methods of evaluation and therapeutic possibilities of acetyl-L-carnitine. Int J Clin Pharmacol Res. 1988;8(5):367-76.
Pettegrew JW, Klunk WE, Panchalingam K, Kanfer JN, McClure RJ. Clinical and neurochemical effects of acetyl-L-carnitine in Alzheimer’s disease. Neurobiol Aging. 1995 Jan-Feb;16(1):1-4.
Postiglione A, Soricelli A, Cicerano U, Mansi L, De Chiara S, Gallotta G, Schettini G, Salvatore M. Effect of acute administration of L-acetyl carnitine on cerebral blood flow in patients with chronic cerebral infarct. Pharmacol Res. 1991 Apr;23(3):241-6.
Pueschel SM. The effect of acetyl-L-carnitine administration on persons with Down syndrome. Res Dev Disabil. 2006 Nov-Dec;27(6):599-604.
Quatraro A, Roca P, Donzella C, Acampora R, Marfella R, Giugliano D. Acetyl-L-carnitine for symptomatic diabetic neuropathy. Diabetologia. 1995 Jan;38(1):123.
Rai G, Wright G, Scott L, Beston B, Rest J, Exton-Smith AN. Double-blind, placebo controlled study of acetyl-l-carnitine in patients with Alzheimer’s dementia. Curr Med Res Opin. 1990;11(10):638-47.
Reid MS, Casadonte P, Baker S, Sanfilipo M, Braunstein D, Hitzemann R, Montgomery A, Majewska D, Robinson J, Rotrosen J. A placebo-controlled screening trial of olanzapine, valproate, and coenzyme Q10/L-carnitine for the treatment of cocaine dependence. Addiction. 2005 Mar;100 Suppl 1:43-57.
Salvioli G, Neri M. L-acetylcarnitine treatment of mental decline in the elderly. Drugs Exp Clin Res. 1994;20(4):169-76.
Sano M, Bell K, Cote L, Dooneief G, Lawton A, Legler L, Marder K, Naini A, Stern Y, Mayeux R. Double-blind parallel design pilot study of acetyl levocarnitine in patients with Alzheimer’s disease. Arch Neurol. 1992 Nov;49(11):1137-41.
Scarpini E, Sacilotto G, Baron P, Cusini M, Scarlato G. Effect of acetyl-L-carnitine in the treatment of painful peripheral neuropathies in HIV+ patients. J Peripher Nerv Syst. 1997;2(3):250-2.
Sima AA, Calvani M, Mehra M, Amato A; Acetyl-L-Carnitine Study Group. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care. 2005 Jan;28(1):89-94.
Spagnoli A, Lucca U, Menasce G, Bandera L, Cizza G, Forloni G, Tettamanti M, Frattura L, Tiraboschi P, Comelli M, et al. Long-term acetyl-L-carnitine treatment in Alzheimer’s disease. Neurology. 1991 Nov;41(11):1726-32.
Tempesta E, Troncon R, Janiri L, Colusso L, Riscica P, Saraceni G, Gesmundo E, Calvani M, Benedetti N, Pola P. Role of acetyl-L-carnitine in the treatment of cognitive deficit in chronic alcoholism. Int J Clin Pharmacol Res. 1990;10(1-2):101-7.
Thal LJ, Calvani M, Amato A, Carta A. A 1-year controlled trial of acetyl-l-carnitine in early-onset AD. Neurology. 2000 Sep 26;55(6):805-10.
Thal LJ, Carta A, Clarke WR, Ferris SH, Friedland RP, Petersen RC, Pettegrew JW, Pfeiffer E, Raskind MA, Sano M, Tuszynski MH, Woolson RF. A 1-year multicenter placebo-controlled study of acetyl-L-carnitine in patients with Alzheimer’s disease. Neurology. 1996 Sep;47(3):705-11.
Uzun N, Sarikaya S, Uluduz D, Aydin A. Peripheric and automatic neuropathy in children with type 1 diabetes mellitus: the effect of L-carnitine treatment on the peripheral and autonomic nervous system. Electromyogr Clin Neurophysiol. 2005 Sep-Oct;45(6):343-51.
Virmani A, Binienda Z. Role of carnitine esters in brain neuropathology. Mol Aspects Med. 2004 Oct-Dec;25(5-6):533-49.
Youle M, Osio M; ALCAR Study Group. A double-blind, parallel-group, placebo-controlled, multicentre study of acetyl L-carnitine in the symptomatic treatment of antiretroviral toxic neuropathy in patients with HIV-1 infection. HIV Med. 2007 May;8(4):241-50.
Zanardi R, Smeraldi E. A double-blind, randomised, controlled clinical trial of acetyl-L-carnitine vs. amisulpride in the treatment of dysthymia. Eur Neuropsychopharmacol. 2006 May;16(4):281-7.