The metabolic fate of alpha linolenic acid (ALA)
Extremely limited conversion efficiency
ABSTRACT:
EPA and DHA possess important physiological and biological properties in human health and development; however, it is alpha-linolenic acid (ALA) that is classified as the essential n-3 PUFA. While humans have the required enzymes to biosynthesize omega-3 long-chain polyunsaturated fatty acids (PUFA), studies demonstrate that the majority of ALA is β-oxidized and only ~5% of ALA is converted to eicosapentaenoic acid (EPA) and <0.5% of ALA is converted to docosahexaenoic acid (DHA). Even very high intakes of dietary ALA fail to effectively modulate plasma and tissue levels of DHA. Furthermore, the abundance of linoleic acid (LA) in our diet, as well as age, gender and genetics influence the conversion efficiency of ALA. Therefore, direct dietary consumption or supplementation of EPA and DHA from fish, fish oil, or algae is the only clinically effective way to increase blood and tissue levels of these longchain PUFA in humans for optimal health and disease prevention.
INTRODUCTION[spacer height=”20px”]
Considerable clinical interest has focused on the health benefits of omega-3 polyunsaturated fatty acids (ω-3 PUFA). While consumption of marine derived eicosapentaenoic acid (EPA; 20:5ω-3) and docosahexaenoic acid (DHA; 22:6ω-3) have been evidenced to prevent cardiovascular and neurological disease risk, the specific function of plant derived alpha-linolenic acid (ALA; 18:3ω-3) remains a matter of debate. EPA and DHA play a vital role in cellular membranes, maintaining fluidity, protein and cellular functions, as well as influencing eicosanoid metabolism, gene expression and cell signaling (Adkins 2010). However, the specific bioactive role of ALA is unclear (Sinclair 2002). It has been suggested that the major function of ALA is to serve as a precursor for EPA and DHA, a pathway that has received much attention and clinical investigation.
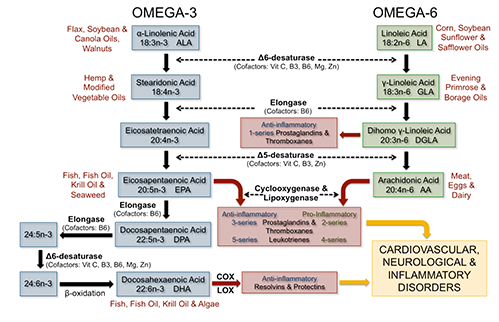
In 1929, Burr and Burr first identified the nutritional essentiality of LA, and later ALA (Burr 1929, Burr 1930). ALA is the parent ω-3 PUFA containing three double bonds with the first double bond located at the third carbon relative to the methyl end of the 18-carbon chain. ALA and ω-6 linoleic acid (LA, 18:2ω-6) are termed essential fatty acids because humans lack the delta (Δ)15- and Δ12-desaturase enzymes required for insertion of a double bond at the ω-3 or ω-6 position, respectively. Since EPA and DHA can be synthesized from ALA via a series of alternating desaturation and elongation steps, these long-chain PUFA are not considered essential. However, whether endogenous synthesis of EPA and DHA from ALA is adequate to support growth, physiological needs, and disease risk reduction is questioned (Harris 2009, Saldanha 2009). Considering negligible plasma and tissue levels of ALA, yet its classification of essential, this review will examine the biosynthesis of EPA and DHA from ALA, factors influencing ALA metabolism, and ω-3 PUFA in the current diet.
BIOSYNTHESIS OF LONG-CHAIN POLYUNSATURATED FATTY ACIDS
The predominate site of ALA desaturation and elongation occurs in the liver, however, also occurs to a lesser extent in other tissues, including the brain and heart (Cho 1999 A, Cho 1999 B). Dietary LA and ALA compete for the same desaturase and elongase enzymes for long-chain PUFA biosynthesis with the majority of this pathway occurring in the endoplasmic reticulum. Of interest, the desaturase enzymes have a higher affinity for ALA versus LA (Plourde 2007), however, high levels of dietary LA saturate Δ6-desaturation inhibiting the accumulation of ω-3 long-chain PUFA, namely EPA (Angela Liou 2009, Liou 2007).
The first reaction in the conversion pathway is the desaturation of ALA to stearidonic acid (SDA; 18:4ω-3) or LA to gamma-linolenic acid (GLA; 18:3ω-6) via the rate-limiting enzyme Δ6-desaturase (Sprecher 2000) (Figure 1). Next, elongation and Δ5-desaturation converts SDA to EPA and GLA to arachidonic acid (AA; 20:4ω-6), the major bioactive n-6 PUFA in tissue membranes and precursor for proinflammatory eicosanoids (Adkins 2010). Compared to AA, EPA is quantitatively a minor fatty acid in tissue membranes and undergoes further elongation to docosapentaenoic acid (DPA; 22:5ω- 3), or is metabolized by cyclooxygenase (COX) and lipoxygenase (LOX) enzymes in the synthesis of anti-inflammatory eicosanoids. In humans, DPA undergoes another elongation and Δ6-desaturation step, and then partial β-oxidation in the peroxisome to form DHA (Sprecher 2000). Structurally, DHA is the predominant ω-3 PUFA esterified into tissue membrane phospholipids. DHA can also be metabolized to produce resolvins and protectins in the resolution of inflammation (Adkins 2010). It has been hypothesized that multiple use of the rate-limiting Δ6-desaturase enzyme for the conversion of ALA to SDA and 24:5n-3 to 24:6n-3 may lead to a “bottle-neck” in the metabolic pathway and an associated decrease in the synthesis of DHA (D’andrea 2002, Kitson 2010). Another possible rate-limiting step may be related to the compartmental translocation of 24:6n-3 from the endoplasmic reticulum to the peroxisome. Both hypotheses demand further investigation.
RESULTS FROM HUMAN STUDIES:
The conversion of ALA to long-chain ω-3 PUFA is very inefficient. Dietary supplementation and isotope tracer studies in humans demonstrate a direct linear relationship between dietary ALA and plasma and tissue concentrations of EPA, at approximately 5% conversion efficiency (Plourde 2007). However, biosynthesis of DHA is negligible with typically less than 0.5% conversion of ALA to DHA. Human studies supplementing ALA ranging from 1.5 to 40 g/day for a minimum of three weeks report a relative increase in phospholipid concentration of EPA ranging from trace levels to 250% (Plourde 2007). Studies have also observed an increase in plasma and tissue concentrations of DPA, although to a lesser extent than EPA concentrations. However, the majority of dietary ALA intervention studies fail to effectively modulate plasma and tissue levels of DHA. Gillingham et al demonstrated that consuming 20 g/day of ALA from a flaxseed oil supplemented diet (~2.5 tbsp flaxseed oil daily) resulted in a relative 222% increase in plasma EPA concentration (1.74% total fatty acids) compared with control (0.54% total fatty acids) (2011). However, DHA concentration did not change after supplementing with flaxseed oil. Similarly, supplementing lactating women with 20 g/day of flaxseed oil (10.7 g/day ALA) for four weeks increased breast milk EPA and DPA, but not DHA concentrations (Francois 2003).
Additionally, very high levels of dietary ALA, as well as LA, may saturate the Δ6-desaturase enzyme further inhibiting the final steps of Δ6-desaturation of 24:5n-3 to DHA (Gibson 2011). Even with vegetarian and vegan diets, ALA conversion to DHA is not up-regulated (Fokkema 2000, Li 1999). James et al revealed that compared to dietary ALA, supplementing with SDA-enriched vegetable oil (therefore, bypassing the first Δ6-desaturase rate-limiting step) more effectively increased plasma and tissue concentrations of EPA, however, did not raise DHA concentrations (2003). In addition, EPA supplementation does not significantly elevate DHA concentrations (Brenna 2009). Therefore, the only effective way to increase tissue DHA concentrations is through its direct consumption.
FACTORS AFFECTING EPA AND DHA BIOSYNTHESIS FROM ALA
DIETARY Ω-6 LINOLEIC ACID CONTENT: The abundance of LA and resulting high ω-6 to ω-3 PUFA ratio in current Western diets significantly impedes the conversion efficiency of ALA. Liou and collegues demonstrated that while maintaining dietary ALA at 1% energy, increasing levels of dietary LA from 4% to 10% energy, thus varying the ω-6 to ω-3 PUFA ratio from 4:1 to 10:1, resulted in a decrease in plasma EPA concentrations in healthy men (Angela Liou 2009, Liou 2007). In another human dietary intervention study, lowering the ω-6 to ω-3 PUFA ratio resulted in a significant decrease in platelet aggregation (Freese 1994). Furthermore, trans fats rich in hydrogenated vegetable oils inhibit the synthesis of ω-3 long-chain PUFA (Kummerow 2004).
MICRONUTRIENTS: Vitamins B3, B6, and C, magnesium and zinc are important cofactors to the Δ5- and Δ6-desaturase enzymes. Therefore, low intakes of these essential micronutrients may lead to a reduction in the synthesis of ω-3 long-chain PUFA (Cunnane 1988, de Lorgeril 2005, Harris 2009).
GENDER: Gender may affect biosynthesis of long-chain PUFA, as DHA concentration of plasma phospholipids have been shown to be higher in women than in men (Decsi 2011). Giltay et al observed a 15% increase in DHA status in women compared with men (2004). Furthermore, administration of oral estradiol increased DHA status by 42%, while testosterone decreased DHA status by 22%. It is proposed that estrogen may upregulate ALA metabolism to DHA, and thus, increase maternal DHA status particularly during pregnancy due to the greater demand of DHA for fetal neurological development (Otto 2001, Innis 2000).
AGE: Studies suggest that infants exhibit increased conversion of ALA to long-chain PUFA, including DHA (Clark 1992, Jensen 1996). A study tracing ALA metabolism in preterm infants fed long-chain PUFA enriched formula reported that in one-month old infants about 42% of ALA was converted to DHA, whereas only 11% was converted at three-months of age, and 7% at seven months of age (Carnielli 2007). Typically, conversion of ALA to DHA is still limited in healthy infants at ~1% (Brenna 2009). However, differences in adult age (18–29 versus 45–69 years) have not been shown to influence metabolism of ALA to EPA or DHA (Patenaude 2009).
GENETICS: Fatty acid desaturase genes FADS1 and FADS2 encode for Δ5-desaturase and Δ6-desaturase, respectively (Cho 1999, Cho 1999). Schaeffer and colleagues published the first study reporting that single nucleotide polymorhpisms (SNP) of the FADS1/FADS2 gene cluster modulate ALA and LA metabolism leading to differences in phospholipid PUFA concentrations (2006). More specifically, minor allele carriers for SNPs located in the FADS1/ FADS2 gene cluster have reduced ability to convert LA to AA (Tanaka 2009) or ALA to EPA (Gillingham 2013a). Of interest, genetic variation explains ~40% or more of the interindividual variability in all fatty acid concentrations (Lemaitre 2008). Martinelli et al reported that individuals carrying FADS polymorphisms, associated with higher conversion of LA to AA, have increased proinflammatory CRP concentrations and risk for coronary artery disease (2008).
OTHER METABOLIC PATHWAYS
β-oxidation accounts for the major metabolic fate of dietary ALA (McCloy 2004). McCloy et al. demonstrated that ~71% of dietary ALA was oxidized over a seven day period, the highest oxidation rate of all fatty acids (2004). During mitochondrial β-oxidation of ALA, carbon units generated in the form of acetyl-CoA can be recycled to synthesize SFA, MUFA, cholesterol and ketone bodies (Burdge 2003). Second to β-oxidation, storage of ALA in adipose tissues accounts for a main metabolic route of dietary ALA (McCloy 2004), however, ALA is readily mobilized from fatty acid tissue during increased energy demands of the body.
DIETARY OMEGA-3 POLYUNSATURATED FAT INTAKE AND RECOMMENDATIONS
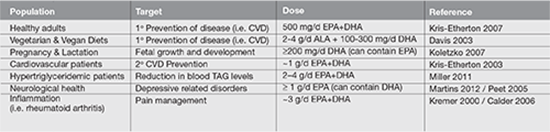
Since ALA and LA are the only fatty acids classified as essential, the US Institute of Medicine’s (IOM) Food and Nutrition Board, together with Health Canada, has established an Adequate Intake (AI, an intake level necessary to achieve nutritional adequacy and prevent deficiency symptoms) for ALA as 1.1-1.6 g/day and for LA as 12-17 g/day for adults (Institute of Medicine 2002). Symptoms of ALA and LA deficiency include scaly dry skin, reproductive failure, numbness, weakness, pain, and blurred vision (Collins 1971, Holman 1982). However, current Western intake of ALA at 1.4-1.8 g/day and LA at 13-18 g/day by adults (Rhodes 2012) exceed the outlined AI by the IOM. The clinical concern is that current intake of combined EPA and DHA at 90–120 mg/day fail to meet recommendations outlined by professional health organization ranging from 500–4000 mg/ day (Table 1). Due to the importance of DHA in fetal retina and brain growth and development, the minimum recommended intake of DHA during pregnancy and lactation is 200 mg/day (Koletzko 2007). However, pregnant and lactating women in Canada and the United States only consume approximately 80 and 50 mg/day, respectively (Denomme 2005, Rhodes 2012). In addition, biosynthesis of long-chain PUFA, namely DHA from dietary ALA is insufficient to meet target recommended intakes of EPA and DHA for optimal health and disease prevention.
The FAO/WHO joint committee recommends an ω-6 to ω-3 ratio between 5:1 and 10:1 (WHO/FAO 1995). Of interest, the Paleolithic diet contained an ω-6 to ω-3 ratio of <1:1 (Kuipers 2010), while current intakes range from 10-25:1 in the North America diet.
SAFETY OF EPA AND DHA SUPPLEMENTATION
In 1997, the US Food and Drug Administration (FDA) granted Generally Recognized As Safe (GRAS) status to refined fish oils and indicated that the consumption of up to 3g/day of EPA+DHA is considered safe for the adults population, including patients with diabetes, bleeding tendencies, and elevated LDL-cholesterol (1997). Intake exceeding 3g/day should only be recommended under the guidance of a healthcare practitioner.
DIETARY SOURCES:
ω-6 LA is abundant in our diet, rich in corn, safflower, soybean, and sunflower oils. However, ω-3 ALA is found in only a limited amount of foods, namely flaxseed, walnuts, soybean and their oils, as well as canola oil, butternuts, and chia seeds. Although flaxseed oil represents the richest source of ALA (7.3 g ALA/tbsp), it is not commonly consumed compared with soybean oil (0.9 g ALA/tbsp) and canola oil (1.3 g ALA/ tbsp) (USDA 2011). Purslane, a wild leafy vegetable common in the Eastern Mediterranean diet, contains 300–400mg ALA/100g serving (Simopoulos 1996).
EPA and DHA are primarily found in fish, fish oil, krill oil or algae. However, the concentration of EPA and DHA substantially varies in fish species (Table 2). For example, fatty fish such as farmed Atlantic salmon provides 1.83 g EPA+DHA per 3 oz serving, whereas the same amount of lean fish such as cod provides only 0.14 g EPA+DHA (USDA 2011). Therefore, one would have to consume approximately four servings of farmed Atlantic salmon or 52 servings of cod per week to achieve a recommended intake of 1g/day (Table 2). Where fatty fish intake exceeds two servings per day, such as Greenland and Japan (Stark 2002, Iso H 1989), plasma EPA/DHA levels are in what is widely considered a therapeutic range (Harris 2010). However, in most parts of the world, theselevels are not being achieved. In addition, depending on the species of fish, excessive fish intake may lead to concerns surrounding mercury exposure, particularly in pregnancy (Koletzko 2007). Taken together, fish oil supplements may provide a more feasible option to target clinical recommendations for EPA+DHA. Minor amounts of EPA can also be found in seaweed, such as kelp, laver, and wakame ranging in EPA levels from 0.004 to 0.186 g/100g serving (USDA 2011). Additionally, microalgae-based supplements offer an alternative for DHA intakes for vegetarians/vegans.
CONCLUSION
The consensus of human clinical trials substantiate that the biosynthesis of EPA and DHA from dietary ALA is extremely limited and insufficient to meet protective tissue levels for disease prevention. In addition, factors including diet, gender, age and genetics affect individual’s capacity for biosynthesis and resulting EPA and DHA tissue concentration. Considering the majority of dietary ALA is β-oxidized, negligible conversion rates, and low intakes of EPA and DHA in the current diet, professional organizations emphasize direct supplementation of EPA and DHA in the diet for optimal health and disease risk reduction.
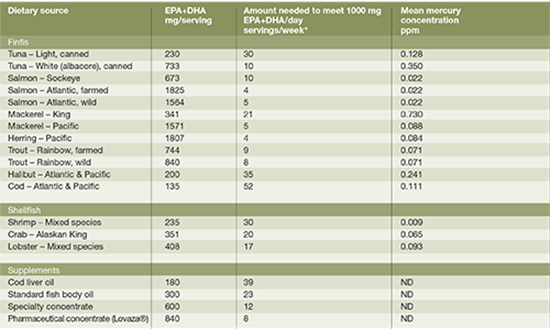
week to provide 1000 mg of EPA+DHA per day.
REFERENCES
Adkins Y, Kelley DS. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem. 2010 Sep;21(9):781-92.
Angela Liou Y, Innis SM. Dietary linoleic acid has no effect on arachidonic acid, but increases n-6 eicosadienoic acid, and lowers dihomo-gamma-linolenic and eicosapentaenoic acid in plasma of adult men. Prostaglandins Leukot Essent Fatty Acids. 2009 Apr;80(4):201-6.
Brenna JT, Salem N,Jr, Sinclair AJ, Cunnane SC, International Society for the Study of Fatty Acids and Lipids, ISSFAL. alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids. 2009 Feb-Mar;80(2-3):85-91.
Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to palmitic, palmitoleic, stearic and oleic acids in men and women. Prostaglandins Leukot Essent Fatty Acids. 2003 Oct;69(4):283-90.
Burr GO, Burr MM. A new deficiency disease produced by the rigid exclusion of fat from the diet. J Biol Chem. 1929(2);82:345-67.
Burr GO, Burr MM. On the nature and role of the fatty acids esential in nutrition. J Biol Chem. 1930(2);86:587-621.
Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006 Jun;83(6 Suppl):1505S-1519S.
Carnielli VP, Simonato M, Verlato G, Luijendijk I, De Curtis M, Sauer PJ, Cogo PE. Synthesis of long-chain polyunsaturated fatty acids in preterm newborns fed formula with long-chain polyunsaturated fatty acids. Am J Clin Nutr. 2007 Nov;86(5):1323-30.
Clark KJ, Makrides M, Neumann MA, Gibson RA. Determination of the optimal ratio of linoleic acid to alpha-linolenic acid in infant formulas. J Pediatr. 1992 Apr;120(4 Pt 2):S151-8.
Cho HP, Nakamura M, Clarke SD. Cloning, expression, and fatty acid regulation of the human delta-5 desaturase. J Biol Chem. 1999A Dec;274(52):37335-9.
Cho HP, Nakamura MT, Clarke SD. Cloning, expression, and nutritional regulation of the mammalian delta-6 desaturase. J Biol Chem. 1999B Jan;274(1):471-7.
Collins FD, Sinclair AJ, Royle JP, Coats DA, Maynard AT, Leonard RF. Plasma lipids in human linoleic acid deficiency. Nutr Metab. 1971;13(3):150-67.
Cunnane SC. Role of zinc in lipid and fatty acid metabolism and in membranes. Prog Food Nutr Sci. 1988;12(2):151- 88.
D’andrea S, Guillou H, Jan S, Catheline D, Thibault JN, Bouriel M, Rioux V, Legrand P. The same rat delta 6-desaturase not only acts on 18- but also on 24-carbon fatty acids in very-long-chain polyunsaturated fatty acid biosynthesis. Biochem J. 2002 May;364(Pt 1):49-55.
Davis BC, Kris-Etherton PM. Achieving optimal essential fatty acid status in vegetarians: current knowledge and practical implications. Am J Clin Nutr. 2003 Sep;78(3 Suppl):640S-646S.
de Lorgeril M, Salen PDefaye P. Importance of nutrition in chronic heart failure patients. Eur Heart J. 2005 Nov;26(21):2215-7.
Decsi T, Kennedy K. Sex-specific differences in essential fatty acid metabolism. Am J Clin Nutr. 2011 Dec;94(6 Suppl):1914S-1919S.
Food and Drug Administration. Mercury levels in commercial fish and shellfish (1990-2010). Washington, DC, 2011. Internet: http://www.fda.gov/Food/FoodSafety/Product-SpecificInformation/Seafood/Foodborne Pathogens Contaminants/Methylmercury/ucm115644.htm.
Fokkema MR, Brouwer DA, Hasperhoven MB, Martini IA, Muskiet FA. Short-term supplementation of low-dose gamma-linolenic acid (GLA), alpha-linolenic acid (ALA), or GLA plus ALA does not augment LCP omega 3 status of Dutch vegans to an appreciable extent. Prostaglandins Leukot Essent Fatty Acids. 2000 Nov;63(5):287-92.
Francois CA, Connor SL, Bolewicz LC, Connor WE. Supplementing lactating women with flaxseed oil does not increase docosahexaenoic acid in their milk. Am J Clin Nutr. 2003 Jan;77(1):226-33.
Freese R, Mutanen M, Valsta LM, Salminen I. Comparison of the effects of two diets rich in monounsaturated fatty acids differing in their linoleic/alpha-linolenic acid ratio on platelet aggregation. Thromb Haemost. 1994 Jan;71(1):73-7.
Gibson RA, Muhlhausler B, Makrides M. Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (LCPUFAs), with a focus on pregnancy, lactation and the first 2 years of life. Matern Child Nutr. 2011 Apr;7 Suppl 2:17-26.
Gillingham LG, Gustafson JA, Han SY, Jassal DS, Jones PJ. High-oleic rapeseed (canola) and flaxseed oils modulate serum lipids and inflammatory biomarkers in hypercholesterolaemic subjects. Br J Nutr. 2011 Feb;105(3):417-27.
Gillingham LG, Harding SV, Rideout TC, Yurkova N, Cunnane SC, Eck PK, Jones PJ. Dietary oils and FADS1-FADS2 genetic variants modulate [13C]α-linolenic acid metabolism and plasma fatty acid composition. Am J Clin Nutr. 2013 Jan;97(1):195-207.(a)
Gillingham LG, Jones PJH. Chapter 4. The Evolution of Omega-3 Fatty Acids in the Human Diet, in The Omega-3 Fatty Acid Deficiency Syndrome: Opportunities for Disease Prevention. Editor: Robert K. McNamara; Nova Science Publishers, Hauppauge, NY, 2013.(b)
Giltay EJ, Gooren LJ, Toorians AW, Katan MB, Zock PL. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr. 2004 Nov;80(5):1167-74.
Harris WS, Mozaffarian D, Lefevre M, Toner CD, Colombo J, Cunnane SC, Holden JM, Klurfeld DM, Morris MC, Whelan J. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr. 2009 Apr;139(4):804S-19S.
Harris WS. The omega-3 index: clinical utility for therapeutic intervention. Curr Cardiol Rep. 2010 Nov;12(6):503-8.
Holman RT, Johnson SB, Hatch TF. A case of human linolenic acid deficiency involving neurological abnormalities. Am J Clin Nutr. 1982 Mar;35(3):617-23.
Innis SM. The role of dietary n-6 and n-3 fatty acids in the developing brain. Dev Neurosci. 2000 Sep-Dec;22(5-6):474-80.
Institute of Medicine. Dietary reference intakes: Energy, carbohydrates, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academies Press, 2002.
Iso H, Sato S, Folsom AR, Shimamoto T, Terao A, Munger RG, Kitamura A, Konishi M, Iida M, Komachi Y. Serum fatty acids and fish intake in rural Japanese, urban Japanese, Japanese American and Caucasian American men. Int J Epidemiol. 1989 Jun;18(2):374-81.
James MJ, Ursin VM, Cleland LG. Metabolism of stearidonic acid in human subjects: comparison with the metabolism of other n-3 fatty acids. Am J Clin Nutr. 2003 May;77(5):1140-5.
Jensen CL, Chen H, Fraley JK, Anderson RE, Heird WC. Biochemical effects of dietary linoleic/alpha-linolenic acid ratio in term infants. Lipids. 1996 Jan;31(1):107-13.
Kitson AP, Stroud CK, Stark KD. Elevated production of docosahexaenoic acid in females: potential molecular mechanisms. Lipids. 2010 Mar;45(3):209-24.
Koletzko B, Cetin I, Brenna JT. Dietary fat intakes for pregnant and lactating women. Br J Nutr. 2007 Nov;98(5):873-7.
Kremer JM. n-3 fatty acid supplements in rheumatoid arthritis. Am J Clin Nutr. 2000 Jan;71(1 Suppl):349S-51S.
Kris-Etherton PM, Harris WS, Appel LJ; AHA Nutrition Committee. American Heart Association. Omega-3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol. 2003 Feb;23(2):151-2.
Kris-Etherton PM, Innis S, Ammerican Dietetic Assocition, Dietitians of Canada. Position of the American Dietetic
Association and Dietitians of Canada: dietary fatty acids. J Am Diet Assoc. 2007 Sep;107(9):1599-611.
Kuipers RS, Luxwolda MF, Dijck-Brouwer DA, Eaton SB, Crawford MA, Cordain L, Muskiet FA. Estimated macronutrient and fatty acid intakes from an East African Paleolithic diet. Br J Nutr. 2010 Dec;104(11):1666-87.
Kummerow FA, Zhou Q, Mahfouz MM, Smiricky MR, Grieshop CM, Schaeffer DJ. Trans fatty acids in hydrogenated fat inhibited the synthesis of the polyunsaturated fatty acids in the phospholipid of arterial cells. Life Sci. 2004 Apr;74(22):2707-23.
Lemaitre RN, Siscovick DS, Berry EM, Kark JD, Friedlander Y. Familial aggregation of red blood cell membrane fatty acid composition: the Kibbutzim Family Study. Metabolism. 2008 May;57(5):662-8.
Li D, Sinclair A, Wilson A, Nakkote S, Kelly F, Abedin L, Mann N, Turner A. Effect of dietary alpha-linolenic acid on thrombotic risk factors in vegetarian men. Am J Clin Nutr. 1999 May;69(5):872-82.
Liou YA, King DJ, Zibrik D, Innis SM. Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J Nutr. 2007 Apr;137(4):945-52.
Martinelli N, Girelli D, Malerba G, Guarini P, Illig T, Trabetti E, Sandri M, Friso S, Pizzolo F, Schaeffer L, et al. FADS genotypes and desaturase activity estimated by the ratio of arachidonic acid to linoleic acid are associated with inflammation and coronary artery disease. Am J Clin Nutr. 2008 Oct;88(4):941-9.
Martins JG, Bentsen H, Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry. 2012 Dec;17(12):1144-9; discussion 1163-7.
McCloy U, Ryan MA, Pencharz PB, Ross RJ, Cunnane SC. A comparison of the metabolism of eighteen-carbon 13C-unsaturated fatty acids in healthy women. J Lipid Res. 2004 Mar;45(3):474-85.
Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris- Etherton PM, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011 May 24;123(20):2292-333.
Otto SJ, van Houwelingen AC, Badart-Smook A, Hornstra G. Changes in the maternal essential fatty acid profile during early pregnancy and the relation of the profile to diet. Am J Clin Nutr. 2001 Feb;73(2):302-7.
Patenaude A, Rodriguez-Leyva D, Edel AL, Dibrov E, Dupasquier CM, Austria JA, Richard MN, Chahine MN, Malcolmson LJ, Pierce GN. Bioavailability of alpha-linolenic acid from flaxseed diets as a function of the age of the subject. Eur J Clin Nutr. 2009 Sep;63(9):1123-9.
Peet M, Stokes C. Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs. 2005;65(8):1051-9.
Plourde M, Cunnane SC. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Appl Physiol Nutr Metab. 2007 Aug;32(4):619-34.
Simopoulos AP, Salem N,Jr. Purslane: a terrestrial source of omega-3 fatty acids. N Engl J Med. 1986 Sep;315(13):83.
Stark KD, Mulvad G, Pedersen HS, Park EJ, Dewailly E, Holub BJ. Fatty acid compositions of serum phospholipids of postmenopausal women: a comparison between Greenland Inuit and Canadians before and after supplementation with fish oil. Nutr. 2002 Jul-Aug;18(7-8):627-30.
Rhodes, D.G., Clemens, J.C., Goldman, J.D., LaComb, R.P., Moshfegh, A.J. 2012. 2009-2010 What We Eat In America, NHANES Tables 1-36. Worldwide Web Site: Food Surveys Research Group. Retrieved from http://www.ars.usda.gov/ Services/docs.htm?docid=18349
Saldanha LG, Salem N,Jr, Brenna JT. Workshop on DHA as a required nutrient: overview. Prostaglandins Leukot Essent Fatty Acids. 2009 Aug-Sep;81(2-3):233-6.
Schaeffer L, Gohlke H, Müller M, Heid IM, Palmer LJ, Kompauer I, Demmelmair H, Illig T, Koletzko B, Heinrich J. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum Mol Genet. 2006 Jun;15(11):1745-56.
Sinclair AJ, Attar–Bashi NM, Li D. What is the role of α–linolenic acid for mammals? Lipids. 2002 Dec;37(12):1113-23.
Sprecher H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim Biophys Acta. 2000 Jul;1486(2-3):219-31.
Tanaka T, Shen J, Abecasis GR, Kisialiou A, Ordovas JM, Guralnik JM, Singleton A, Bandinelli S, Cherubini A, Arnett D, et al. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PLoS Genet. 2009 Jan;5(1):e1000338.
US Food and Drug Administration, Department of Health and Human Services. Substances affirmed as generally recognized as safe: menhaden oil. 1997. Retrieved from http://www.gpo.gov/fdsys/ pkg/FR-1997-06-05/html/97-14683.htm
USDA (US Department of Agriculture). National nutrient database 2011. Retrieved from http://www.nal.usda.gov/fnic/ foodcomp/search/ WHO/FAO joint consultation.
WHO and FAO joint consultation: fats and oils in human nutrition. Nutr Rev. 1995 Jul;53(7):202-5.









