Liver Cirrhosis Naturopathic strategies
Abstract
Liver injury exists on a continuum from transiently elevated liver enzymes, to fatty deposits for example in non-alcoholic steatohepatitis (NASH), to liver fibrosis, which is the beginning of liver cirrhosis. Once the process has begun, liver cirrhosis can progress asymptomatically for years until the point where a critical threshold is reached, and remaining hepatocytes can no longer compensate for lost function – decompensated liver disease. This article reviews staging of liver cirrhosis, and focuses on naturopathic treatment strategies than can maximize liver function in cases of moderate to advanced cirrhosis as well as hepatic encephalopathy (HE). These strategies include adequate dietary intake of calories and protein, and restriction of sodium. Nutritional supplementation strategies include branched chain amino acids, probiotics, acetyl-L-carnitine, zinc, silymarin, LOLA (L-ornithine, L-aspartate), and phosphatidylcholine. This paper reviews the evidence supporting these interventions as well as dosing recommendations based on the literature.
Introduction
Cirrhosis is the 12th leading cause of death in the United States, accounting for almost 30 thousand deaths per year (Starr 2011). The leading causes of cirrhosis include chronic alcohol abuse and viral hepatitis; however cirrhosis is the common end pathway of several types of liver injury, including non-alcoholic fatty liver disease (NAFLD), autoimmune hepatic or biliary disease, and obstructive tumors of the liver or biliary ducts (Starr 2011). Liver cirrhosis or “scarring” leads to gradual loss of liver function and the eventual emergence of decompensated liver disease, including hepatic encephalopathy and ascites. This paper will discuss naturopathic strategies that can assist in the management of cirrhosis, particularly decompensated cirrhosis. Intervention with a selection of natural agents offers the potential for delaying and/ or alleviating some of the symptoms associated with this condition, for which conventional treatments remain limited.
Pathophysiology
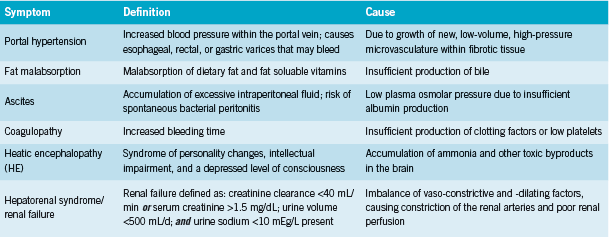
Cirrhosis is “a late stage of hepatic fibrosis that has resulted in widespread distortion of normal hepatic architecture… characterized by regenerative nodules surrounded by dense fibrotic tissue” (Merck 2011). The replacement of functional hepatocytes with non-functioning fibrous tissue results in progressive loss of liver function. Table 1 describes the sequelae of advanced cirrhosis. According to Wolf, these arise from three common underlying malfunctions: “decreased hepatic synthetic function (eg, coagulopathy), decreased detoxification capabilities of the liver (eg, hepatic encephalopathy), [and] portal hypertension (eg, variceal bleeding)” (2011).
Conventional treatment
Cirrhosis is non-reversible, and current treatments are supportive in nature (Merck 2011). These include lactulose to promote bowel elimination of ammonia; neomycin or rifaximin, antibiotics that decreased ammonia producing bacteria in the gut; and salt restrction, diuretics, and paracentesis (peritoneal “tap” procedure with fluid removal) if necessary for ascites (Foster 2010, Starr 2011). The last resort for liver failure is an organ transplant (Merck 2011).
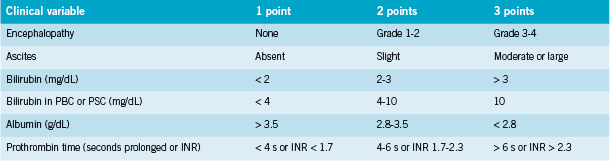
Cirrhosis is staged using the Child-Turcotte-Pugh Scoring System (See Table 2). Severity is scored as (Merck 2011):
• Child Class A=5-6 points (1y survival 100%);
• Child Class B=7-9 points (1y survival 80%);
• Child Class C=10-15 points (1y survival 45%)
Hepatic encephalopathy (HE) ranges from minimal encephalopathy (grade 1-2) to coma (grade 4) in end stage liver disease (ESLD). Minimal encephalopathy is present in 30-84% of patients with cirrhosis (Chadalavada 2010); patients may have an apparently normal mental status, with abnormalities detected only with psychometric testing, but may have intermittent episodes of worsened encephalopathy that may be triggered by infection, diuretic therapy, hypovolemia, renal failure, GI bleeding, infection, and constipation (Chadalavada 2010, Wolf 2011). Certain pscyhoactive medications may also worsen HE (Wolf 2011). HE is graded symptomatically (Merck 2011):
• Grade 1) Sleep disturbance; impaired concentration; depression, anxiety, or irritability
• Grade 2) Drowsiness, disorientation, poor short-term memory; uninhibited behaviour
• Grade 3) Somnolence, confusion, amnesia, anger, paranoia, bizarre behaviour
• Grade 4) Coma
Dietary Guidelines
The goals of dietary treatment for cirrhosis are three-fold:
1) Maintain adequate caloric intake (30-35 kcal/kg dry weight = 1800 kcal/ 60kg person) (Bemeur 2010). Patients with cirrhosis are often undernourished due to anorexia and poor nutrient absorption. Recommendations for dietary composition are 50-60% of calories as carbohydrate; 20-30% as protein; and 10-20% as fat (Chadalavada 2010).
2) Adequate protein intake (1.2 – 1.5 g/kg protein daily) (Bemeur 2010). Traditionally, a low-protein diet (0-40g/d) has been advocated, since ammonia is derived from protein. Recently there is a growing consensus that this strategy is counterproductive. Inducing a state of protein and/ or calorie malnutrition results in catabolism of muscle protein, and has the double effect of increasing ammonia and causing malnutrition & loss of muscle mass; and patients with cirrhosis have increased protein requirements (Cabral 2011). Instead protein restriction should be limited to a small number of protein-intolerant patients in grade 3-4 HE, if there is a lack of response to other therapies, and only for short periods of time (Bemeur 2010). Wolf says, “the vast majority of patients can tolerate a protein-rich diet (>1.2 g/kg/d) including well-cooked chicken, fish, vegetable protein, and, if needed, protein supplements” (2011).
Recent studies have shown that protein intake is safe and has little impact on HE (Cordoba 2004), and a trial by Gheorghe found that a casein-vegetable-based, high-protein high-calorie (HPHC) diet decreased serum ammonia levels and resulted in improved mental status in patients with overt HE (2005).
3) Salt restriction. To minimize sodium/ water retention and ascites, an initial limit of <2000mg sodium/d is utilized; if this is ineffective, a limit of <500mg/d is used.
Dietary changes (eg. fibre intake) to ensure regular elimination through the gut should also be implemented.
Supplemental Interventions
Select nutritional supplements have been shown to improve liver function and decrease ammonia levels, resulting in clinical improvements in patients with HE.
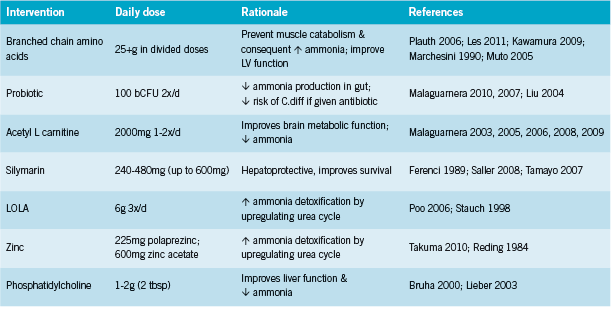
Branched chain amino acids (leucine, isoleucine, valine) are probably the best- studied supplemental intervention for HE. Although their use has been debated, the discussion seems to have settled in favour of BCAAs (Cabral 2011). The European Society for Parenteral and Enteral Nutrition (ESPEN) has strengthened its recommendation for BCAAs in decompensated liver cirrhosis to grade B (Plauth 2006). BCAAs promote protein synthesis and decrease blood ammonia levels (Cabral 2011); compete with aromatic amino acids (phenylalanine, tyrosine, tryptophan) for uptake into the CNS, where these are thought to contribute to HE (Cabral 2011, Iwasa 2003); stimulate human growth factor (HGF), which promotes hepatic regeneration; and there is some indication that they might lower risk of hepatocellular carcinoma (Kobayashi 2008). Clinical trials have shown that BCAA administration improves neurophysiological function and psychometric testing in HE (Egberts 1986, Higuchi 1994, Plauth 1993). In a randomized double blind trial, Marchesini found that BCAA supplemented at 0.24 g/kg (approx. 14g for a 60kg person) for three months rapidly improved neuropsychologic function, and resulted in mild improvement in nutritional parameters and liver function tests (1990).
Kawamura found that in patients with early cirrhosis (Child class A), administration of 12.45g/d BCAAs for 3.2y resulted in significantly slower changes in markers of progression (2009). In addition, BCAAs lowered the incidence of overall major cirrhotic complications to 14.8% (4 of 27 patients) compared to 30.4% (7 of 23 patients) in the control group at 3 years (P = 0.043); potentially prolonging the waiting period until liver organ transplantation is necessary (Kawamura 2009). Similar findings have been corroborated in other studies, where 12g/d BCAAs for 2y reduced risk of the following complications by 33% (HR 0.67, 95% CI 0.49-0.93): death by any cause, development of liver cancer, rupture of esophageal varices, or progress of hepatic failure (Muto 2005).
Conversely, Les et al found that administration of 30g BCAAs daily for approximately one year to 116 patients resulted in improvements in symptoms of minimal HE, but did not reduce rates of recurrence for having an episode of acute HE (2011). This may be in part due to the multifactorial nature of the influences on acute HE (see above sections). BCAAs as part of a more comprehensive nutritional and lifestyle approach might be of benefit in this respect.
Probiotics. Administration of probiotic bacteria has been shown to reduce ammonia levels by competing with urease- producing species such as Klebsiella and Proteus species (Pereg 2011). Malaguarnera found that compared to lactulose treated patients, those treated with Bifidobacterium had significantly improved mental functioning and lower ammonia levels (2010, 2007). Liu found that probiotics plus fiber lowered ammonia levels and improved Child-Turcotte-Pugh functional class in nearly 50% of cases with minimal HE (2004).
Acetyl L carnitine In advanced HE, use of intravenous acetyl L carnitine (ALC) in addition to BCAA has been shown to benefit neurological status (Glasgow coma scale); EEG parameters; serum ammonia; and improve HE grade from 4 to 3 in some patients (54% in one study) compared to BCAA alone (Malaguarnera 2006, 2009).
In earlier stages, oral ALC has shown benefit on neurophysiological function tests, prothrombin time (P < 0.001), bilirubin serum levels (P < 0.01), AST (P < 0.001), fasting serum ammonia levels (P < 0.001), and a significant increase in albumin serum levels (P < 0.005) (Malaguarnera 2008). Although the mechanism by which ALC exerts its effects in HE is unclear, carnitine is known to participate in ketone body production, facilitate mitochondrial function through transfer of acetylCoA, and aid production of acetylcholine (Shores 2008).
Zinc supplementation (225mg zinc acetate) for six months significantly decreased HE grade and blood ammonia levels (P = 0.03 and P = 0.01), and improved Child-Pugh score and neuropsychological tests compared with standard therapy (P = 0.04 and P = 0.02) in patients with grade 1 or 2 recurrent episodic HE unresponsive to standard therapies (lactulose and a proteinrestricted diet) (Takuma 2010). A smaller cross-over trial of 15 patients found lack of effect from 600mg zinc sulfate (Riggio 1991), while an earlier randomized double-blind trial of 22 HE patients did find benefit (Reding 1984). Authors postulated that zinc “probably improved hepatic encephalopathy by correcting the zinc deficiency that compromises conversion of ammonia to urea” (Reding 1984). Although these studies utilize very high dosages, it is possible that more modest dosages (~30mg) may also be of benefit.
Silymarin has not been studied specifically for HE, however it has been studied extensively as a hepatoprotective for liver cirrhosis. A randomized double blind placebo controlled trial of silymarin 140mg three times daily in patients with alcoholic liver cirrhosis found that silymarin significantly prolonged survival: 4-year survival rate 58% in silymarin-treated patients and 39% in the placebo group (P = 0.036) (Ferenci 1989). A more recent meta analysis by Saller including 19 trials found a lack of evidence of silymarin on progression of viral hepatitis, but a reduction in AST (p = 0.01) in alcoholic liver disease in silymarin-treated patients compared with placebo (2008). In liver cirrhosis, total mortality was 16.1% with silymarin and 20.5% with placebo (non-significant), and liver-related mortality was 10.0% with silymarin vs. 17.3% with placebo(p = 0.01) (Saller 2008).
LOLA (L-ornithine, L-aspartate) is another intervention that has been demonstrated to lower ammonia and improve neurological function (Poo 2006). LOLA stimulates the urea cycle and glutamine synthesis which promote ammonia detoxification (Rees 2000).
Phosphatidylcholine Although better studied for NAFLD, a single study reports benefits with use of 2.0g daily of an intravenous formula of “essential phospholipids” including phosphatidylcholine (PC) in patients with stage 3-4 HE (Bruha 2000). Ammonia reductions of ~50% were reported in the treatment group but none in the control group, and survival time was longer in the treatment group: 50.3 versus 34.7 days. A second study reports improvements in liver function (AST, bilirubin) with 1.5g polyenylphosphatidylcholine in subgroups of patients out of a cohort of veterans with chronic alcohol abuse; the groups that benefited most were those with hepatitis C and heavy drinkers (Lieber 2003). It is possible that oral PC may also be of benefit in earlier stages of HE/ cirrhosis.
Other agents for which evidence specific to cirrhosis/ HE is lacking at present, but which may be of benefit based on their mechanism of action include alpha lipoic acid, N-acetyl-cysteine, eicosapentanoic acid, and betaine.
Conclusion
Liver cirrhosis severity ranges from having little or no symptoms (compensated disease), to having minimal-tosevere HE, ascites, and other complications as the pathology progresses. Conventional treatment for cirrhosis and HE remain limited. A selection of natural interventions has shown promise in ameliorating symptoms associated with HE when present, and potentially delaying the onset and progression of HE and cirrhosis, respectively in earlier stages. In addition to specific dietary guidelines, these agents include BCAAs, probiotics, acetyl L carnitine, silymarin, zinc, LOLA, and phosphatidylcholine.
References
Bémeur C, Desjardins P, Butterworth RF. Role of nutrition in the management of hepatic encephalopathy in end-stage liver failure. J Nutr Metab. 2010;2010:489823.
Brůha R, Marecek Z. [Essential phospholipids in the treatment of hepatic encephalopathy]. Vnitr Lek. 2000 Apr;46(4):199-204.
Cabral CM, Burns DL. Low-protein diets for hepatic encephalopathy debunked: let them eat steak. Nutr Clin Pract. 2011 Apr;26(2):155-9.
Chadalavada R, Sappati Biyyani RS, Maxwell J, Mullen K. Nutrition in hepatic encephalopathy. Nutr Clin Pract. 2010 Jun;25(3):257-64.
Córdoba J, López-Hellín J, Planas M, Sabín P, Sanpedro F, Castro F, Esteban R, Guardia J. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004 Jul;41(1):38-43.
Egberts EH, Schomerus H, Hamster W, Jürgens P. [Branched-chain amino acids in the treatment of latent porto-systemic encephalopathy. A placebo-controlled doubleblind cross-over study]. Z Ernahrungswiss. 1986 Mar;25(1):9-28.
Ferenci P, Dragosics B, Dittrich H, Frank H, Benda L, Lochs H, Meryn S, Base W, Schneider B. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989 Jul;9(1):105-13.
Foster KJ, Lin S, Turck CJ. Current and emerging strategies for treating hepatic encephalopathy. Crit Care Nurs Clin North Am. 2010 Sep;22(3):341-50.
Gheorghe L, Iacob R, Vădan R, Iacob S, Gheorghe C. Improvement of hepatic encephalopathy using a modified high-calorie high-protein diet. Rom J Gastroenterol. 2005 Sep;14(3):231-8.
Higuchi K, Shimizu Y, Nambu S, Miyabayashi C, Takahara T, Saito S, Hioki O, Kuwabara Y, Watanabe A. Effects of an infusion of branched-chain amino acids on neurophysiological and psychometric testings in cirrhotic patients with mild hepatic encephalopathy. J Gastroenterol Hepatol. 1994 Jul-Aug;9 (4):366-72.
Iwasa M, Matsumura K, Watanabe Y, Yamamoto M, Kaito M, Ikoma J, Gabazza EC, Takeda K, Adachi Y. Improvement of regional cerebral blood flow after treatment with branched-chain amino acid solutions in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2003 Jul;15(7):733-7.
Kawamura E, Habu D, Morikawa H, Enomoto M, Kawabe J, Tamori A, Sakaguchi H, Saeki S, Kawada N, Shiomi S. A randomized pilot trial of oral branched-chain amino acids in early cirrhosis: validation using prognostic markers for pre-liver transplant status. Liver Transpl. 2009 Jul;15(7):790-7.
Kobayashi M, Ikeda K, Arase Y, Suzuki Y, Suzuki F, Akuta N, Hosaka T, Murashima N, Saitoh S, Someya T, Tsubota A, Kumada H. Inhibitory effect of branched-chain amino acid granules on progression of compensated liver cirrhosis due to hepatitis C virus. J Gastroenterol. 2008;43(1):63-70.
Les I, Doval E, García-Martínez R, Planas M, Cárdenas G, Gómez P, Flavià M, Jacas C, Mínguez B, Vergara M, Soriano G, Vila C, Esteban R, Córdoba J. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol. 2011 Jun;106(6):1081-8.
Lieber CS, Weiss DG, Groszmann R, Paronetto F, Schenker S; Veterans Affairs Cooperative Study 391 Group. II. Veterans Affairs Cooperative Study of polyenylphosphatidylcholine in alcoholic liver disease. Alcohol Clin Exp Res. 2003 Nov;27(11):1765-72.
Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004 May;39(5):1441-9.
Loguercio C, Del Vecchio Blanco C, Coltorti M. Enterococcus lactic acid bacteria strain SF68 and lactulose in hepatic encephalopathy: a controlled study. J Int Med Res. 1987 Nov-Dec;15(6):335-43.
Malaguarnera M, Gargante MP, Malaguarnera G, Salmeri M, Mastrojeni S, Rampello L, Pennisi G, Li Volti G, Galvano F. Bifidobacterium combined with fructo-oligosaccharide versus lactulose in the treatment of patients with hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2010 Feb;22(2):199-206.
Malaguarnera M, Risino C, Cammalleri L, Malaguarnera L, Astuto M, Vecchio I, Rampello L. Branched chain amino acids supplemented with L-acetylcarnitine versus BCAA treatment in hepatic coma: a randomized and controlled double blind study. Eur J Gastroenterol Hepatol. 2009 Jul;21(7):762-70.
Malaguarnera M, Gargante MP, Cristaldi E, Vacante M, Risino C, Cammalleri L, Pennisi G, Rampello L. Acetyl-L-carnitine treatment in minimal hepatic encephalopathy. Dig Dis Sci. 2008 Nov;53(11):3018-25.
Malaguarnera M, Greco F, Barone G, Gargante MP, Malaguarnera M, Toscano MA. Bifidobacterium longum with fructo-oligosaccharide (FOS) treatment in minimal hepatic encephalopathy: a randomized, double-blind, placebo-controlled study. Dig Dis Sci. 2007 Nov;52(11):3259-65.
Malaguarnera M, Pistone G, Astuto M, Vecchio I, Raffaele R, Lo Giudice E, Rampello L. Effects of L-acetylcarnitine on cirrhotic patients with hepatic coma: randomized double-blind, placebo-controlled trial. Dig Dis Sci. 2006 Dec;51(12):2242-7.
Malaguarnera M, Pistone G, Elvira R, Leotta C, Scarpello L, Liborio R. Effects of L-carnitine in patients with hepatic encephalopathy. World J Gastroenterol. 2005 Dec 7;11(45):7197-202.
Malaguarnera M, Pistone G, Astuto M, Dell’Arte S, Finocchiaro G, Lo Giudice E, Pennisi G. L-Carnitine in the treatment of mild or moderate hepatic encephalopathy. Dig Dis. 2003;21(3):271-5.
Marchesini G, Dioguardi FS, Bianchi GP, Zoli M, Bellati G, Roffi L, Martines D, Abbiati R. Long-term oral branched-chain amino acid treatment in chronic hepatic encephalopathy. A randomized double-blind casein-controlled trial. The Italian Multicenter Study Group. J Hepatol. 1990 Jul;11(1):92-101.
The Merck Manual of Diagnosis and Therapy, Nineteenth Edition. Cirrhosis. Ed. Eldon A. Shaffer. Updated 2011. http://www.merckmanuals.com/professional/hepatic_
and_biliary_disorders/fibrosis_and_cirrhosis/cirrhosis.html Accessed 14 April 2012.
Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S, Kumada H; Long-Term Survival Study Group. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005 Jul;3(7):705-13.
O’Keefe SJ, Ogden J, Dicker J. Enteral and parenteral branched chain amino acidsupplemented nutritional support in patients with encephalopathy due to alcoholic liver disease. JPEN J Parenter Enteral Nutr. 1987 Sep-Oct;11(5):447-53.
Olson JC, Wendon JA, Kramer DJ, Arroyo V, Jalan R, Garcia-Tsao G, Kamath PS. Intensive care of the patient with cirrhosis. Hepatology. 2011 Nov;54(5):1864-72. doi: 10.1002/hep.24622.
Pereg D, Kotliroff A, Gadoth N, Hadary R, Lishner M, Kitay-Cohen Y. Probiotics for patients with compensated liver cirrhosis: a double-blind placebo-controlled study. Nutrition. 2011 Feb;27(2):177-81.
Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J; DGEM (German Society for Nutritional Medicine), Ferenci P, Holm E, Vom Dahl S, Müller MJ, Nolte W; ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr. 2006 Apr;25(2):285-94.
Plauth M, Egberts EH, Hamster W, Török M, Müller PH, Brand O, Fürst P, Dölle W. Long-term treatment of latent portosystemic encephalopathy with branched-chain amino acids. A double-blind placebo-controlled crossover study. J Hepatol. 1993 Mar;17(3):308-14.
Poo JL, Góngora J, Sánchez-Avila F, Aguilar-Castillo S, García-Ramos G, Fernández- Zertuche M, Rodríguez-Fragoso L, Uribe M. Efficacy of oral L-ornithine-L-aspartate in cirrhotic patients with hyperammonemic hepatic encephalopathy. Results of a randomized, lactulose-controlled study. Ann Hepatol. 2006 Oct-Dec;5(4):281-8.
Reding P, Duchateau J, Bataille C. Oral zinc supplementation improves hepatic encephalopathy. Results of a randomised controlled trial. Lancet. 1984 Sep 1;2(8401):493-5.
Rees CJ, Oppong K, Al Mardini H, Hudson M, Record CO. Effect of L-ornithine- L-aspartate on patients with and without TIPS undergoing glutamine challenge: a double blind, placebo controlled trial. Gut. 2000 Oct;47(4):571-4.
Riggio O, Ariosto F, Merli M, Caschera M, Zullo A, Balducci G, Ziparo V, Pedretti G, Fiaccadori F, Bottari E, et al. Short-term oral zinc supplementation does not improve chronic hepatic encephalopathy. Results of a double-blind crossover trial. Dig Dis Sci. 1991 Sep;36(9):1204-8.
Saller R, Brignoli R, Melzer J, Meier R. An updated systematic review with metaanalysis for the clinical evidence of silymarin. Forsch Komplementmed. 2008 Feb;15(1):9-20. Sherman KE. Advanced liver disease: what every hepatitis C virus treater should know. Top Antivir Med. 2011 Aug-Sep;19(3):121-5.
Shores NJ, Keeffe EB. Is oral L-acyl-carnitine an effective therapy for hepatic encephalopathy? Review of the literature. Dig Dis Sci. 2008 Sep;53(9):2330-3. Starr SP, Raines D. Cirrhosis: diagnosis, management, and prevention. Am Fam Physician. 2011 Dec 15;84(12):1353-9.
Stauch S, Kircheis G, Adler G, Beckh K, Ditschuneit H, Görtelmeyer R, Hendricks R, Heuser A, Karoff C, Malfertheiner P, Mayer D, Rösch W, Steffens J. Oral L-ornithine- L-aspartate therapy of chronic hepatic encephalopathy: results of a placebo-controlled double-blind study. J Hepatol. 1998 May;28(5):856-64.
Takeshita S, Ichikawa T, Nakao K, Miyaaki H, Shibata H, Matsuzaki T, Muraoka T, Honda T, Otani M, Akiyama M, Miuma S, Ozawa E, Fujimito M, Eguchi K. A snack enriched with oral branched-chain amino acids prevents a fall in albumin in patients with liver cirrhosis undergoing chemoembolization for hepatocellular carcinoma. Nutr Res. 2009 Feb;29(2):89-93.
Takuma Y, Nouso K, Makino Y, Hayashi M, Takahashi H. Clinical trial: oral zinc in hepatic encephalopathy. Aliment Pharmacol Ther. 2010 Nov;32(9):1080-90.
Tamayo C, Diamond S. Review of clinical trials evaluating safety and efficacy of milk thistle (Silybum marianum [L.] Gaertn.). Integr Cancer Ther. 2007 Jun;6(2):146-57.
Wolf, D. Cirrhosis. Medscape Reference. Updated Sept 22 2011. http://emedicine. medscape.com/article/185856-overview Accessed 14 April 2012









