Galectin-3
An emerging treatment target; role of MCP
Chronic illnesses such as cancer, cardiovascular disease, diabetes and others share common metabolic and inflammatory derangements. Novel epidemiological forecasts predict that a new category of diseases may soon be established, classifying these chronic illnesses as “Elevated Galectin-3 Diseases.”
Galectin-3 is a beta-galactoside binding lectin, ie, a carbohydrate binding protein with an affinity for beta-galactoside containing glycans (Iurisci 2000), expressed by epithelial and immune cells (Yu 2010). Several ligands for galectin-3 have been described, including lysosomal-associated membrane proteins 1 and 2, IgE, laminin & fibronectin, and Mac-2 BP (aka 90K) (Iurisci 2000, Yu 2010); and the biological function of galectin-3 is the subject of increasing research. Although the molecule is normally present in the body at relatively low concentrations, levels are greatly increased in a variety of chronic diseases, granting galectin-3 utility as a marker of disease risk and progression (Iurisci 2000). Notably, galectin-3 appears to be an active biomarker, as opposed to other surrogates such as C-reactive protein (CRP).
Galectin-3 has been shown to be involved in a large number of biological processes, including inflammation, proliferation, and fibrosis. In cardiovascular disease, it is implicated in mediating fibrosis (Lin 2009), while in cancer it appears to be active in promoting metastasis and angiogeneisis (Iurisci 2000). For instance, galectin-3 on the cell surface promotes interactions with glycans on adjacent cell surfaces, promoting tumor cell adhesion, invasion, and dissemination (Yu 2011). Conversely, suppression of galectin-3 expression in animal models results in reduced tumor growth and metastasis (Honjo 2001). Circulating galectin-3 may also play a role in the inhibition of anti-tumor T-cell activity (Peng 2008).
In 2011, the groundbreaking PREVEND study (Prevention of REnal and Vascular ENd-stage Disease) found that elevated serum galectin-3 predicted a two-fold increase in all cause mortality among the general population (deBoer 2011, 2012). A total of 7968 people were following for ten years, with overall mortality being 15.6% in the highest quintile, compared to 7.7% in the lowest quintile (De Boer 2011, 2012). For each standard deviation increase in galectin-3, there was a corresponding 46% increase risk in all cause mortality, HR 1.46 (95% CI 1.37-1.56, unadjusted), and this remained upon adjustment for age, gender, and classical risk factors (but not hs-CRP), HR 1.09 (1.01-1.19, p=0.036) (deBoer 2012).
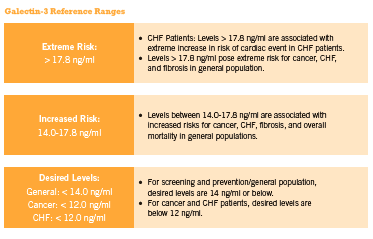
Galectin-3 can be expressed in the nucleus, cytoplasm, mitochondrion, cell surface, extracellular space, circulate freely in the blood stream (Brown 2012), and can be measured in blood samples as well as in tumor tissue samples. Normal serum levels range from 5.4-26.2 ng/mL in 95% of the population based on an analysis conducted by the BGM lab on 1099 samples from healthy patients (BG Medicine 2011). Recently, a blood test for galectin-3 has been approved by the FDA for use in assessing prognosis in patients with chronic heart failure (FDA 2012).
The Role of Galectin-3 in Chronic Disease Processes Cancer
Galectin-3 is over-expressed on the surface of cancer cells (Saleh 2009), where it affects tumor growth through several mechanisms. First, galectin-3 acts as a cell surface adhesion molecule that allows cancer cells to aggregate (Tinari 2001). Second, galectin-3 promotes the dissemination of cancer through the circulatory system, representing an important mechanism for cancer metastasis (Wang 2012). Finally, galectin-3 is also involved in angiogenesis (Nangia-Makker 2000, Yu 2007, Wang 2009, Zhao 2009).
Evaluation of galectin-3 is gaining recognition as an assessment tool in oncology, in particular as a diagnostic marker and as a prognostic marker for different cancers (Brown 2012, Chiu 2010). Studies of galectin-3 in oncology to date have focused predominantly but not exclusively on levels of galectin-3 expression in tumor tissue, rather than on circulating galectin-3, which is the basis of the test in heart failure. Tumor expression of galectin-3 may be elevated or suppressed depending on the cancer type and/ or stage; ie., “decreased expression of galectin-3 … detected in breast cancer, colonic cancer, prostate cancer and head and neck cancer compared to that of corresponding normal tissues, [and increased] galectin-3 expression … in pancreatic, vulvar and colonic carcinomas compared to normal tissues” (Brown 2012). More research on the topic is anticipated to be published in the very near future, however, studies of circulating galectin-3 thus far demonstrate an association with increased risk of cancer, and particularly with increased risk of metastasis. The reason for the apparent lack of consistency in effect between tissue expression levels and circulating levels in not known at this time.
As reported above, the PREVEND study found increased all cause mortality associated with circulating galectin-3 (deBoer 2012); for cancer specific mortality, there was a 41% increased risk among the highest quintile, HR 1.41 (1.28-1.56), unadjusted; though this relationship became insignificant after adjustment.
Iurisci et al examined circulating galectin-3 in 99 cancer patients compared with 50 healthy subjects, finding that levels were significantly higher in cancer patients (p=0.014) (2000). Interestingly, when breast cancer patients only (n=35) were compared to normal subjects, there was no significant difference in galectin-3 levels, however, patients with metatstatic breast cancer had significantly higher galectin-3 compared to those with non-metastatic disease (p<0.032) (Iurisci 2000). Similar results were found for patients with gastrointestinal (GI) cancer (n=25), non-samll cell lung cancer (n=26). Among healthy subjects, median levels were 62ng/mL (range 20-313, 95th percentile 184.3 ng/mL), while maximum levels were found among patients with metastatic GI cancers: median 320ng/mL, range 20-950m/L) (Iurisci 2000).
Saussez et al evaluated circulating galectin-3 in 102 patients with head and neck squamous cell carcinomas (HNSCCs) (2008). Galectin-3 levles were significantly higher in cancer patients compared to controls, median 3.2 and 2.39 ng/mL, respectively (p=0.03), and a threshold value of 4.3 ng/mL enabled discrimination between groups at 90% specificity and 36% sensitivity (Saussez 2008). Notably, galectin-3 levels were significantly higher among five patients with metastatic disease compared with the 97 with localized tumors. Further analysis showed a “weak, but nevertheless significant, prognostic value in terms of periods of survival for HNSCC patients” based on being over or under 4.3 ng/mL (Saussez 2008). Finally, levels of galectin-3 were found to decrease in response to treatment (chemo and/ or radiation and/ or surgery) of the primary tumor, compared to pre-treatment (p=0.001).
Other members of the galectin family have been found to be elevated in patients with breast and colorectal cancer (Barrow 2011).
More research is clearly needed to better evaluate the best use of this test in routine practice, however in the meantime galectin-3 holds immense potential as a cancer prognostic marker. In addition, human trials monitoring the effect on galcetin-3 levels in response to cancer treatment are currently lacking (Pieters 2006), however this presents an intriguing area for future research. It is also worth noting that there is little report in the medical literature of any pharmaceutical galectin-3 inhibitors; instead, the primary agent discussed in review articles thus far has been a natural agent, modified citrus pectin (Pieters 2006).
Cardiovascular Disease
As mentioned above, the galectin-3 blood test has recently received FDA approval as a prognostic test for chronic heart failure (2012). Several large studies have shown that elevated levels of plasma galectin-3 are significantly associated with higher risk of death in patients with acute decompensated and chronic heart failure (De Boer 2011a, DeBoer 2011b, Lok 2010, Shah 2010).
In the CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure) study, 1492 patients with ischaemic systolic heart failure were found to differentially respond to statin drugs according to their levels of galectin-3 (Gullestad 2010). Among patients who received rouvastatin, those whose plasma galectin-3 was below the median (≤19.0 ng/mL) had a 1) lower event rate [hazard ratio (HR) 0.65; 95% confidence interval (CI), 0.46-0.92; P= 0.014] and 2) lower total mortality (HR 0.70; 95% CI, 0.50-0.98; P= 0.038) compared with placebo. Notably, there was no benefit from rouvastatin in patients with higher levels of galectin-3.
Another key study was the HF-ACTION study (Felker 2012). In this study, galectin-3 levels were analyzed in 895 patients with chronic heart failure caused by left ventricular systolic dysfunction. Higher galectin-3 levels were associated with measures of heart failure severity, including “higher New York Heart Association class, lower systolic blood pressure, higher creatinine, higher amino-terminal proB-type natriuretic peptide (NTproBNP), and lower maximal oxygen consumption” (Felker 2012).
Cardiac fibrosis is gaining significant attention as an important risk factor in cardiac disease, in particular chronic heart failure (CHF) (deFilippi 2010), and galectin-3 plays a central role in mediating fibrosis (Liu 2009). At a site of injury/inflammation, galectin-3 is secreted into the extracellular space, activating resting fibroblasts into matrix-producing fibroblasts, thus promoting fibrosis (Liu 2009). Galectin-3 has also been found to beneficially impact other mechanisms associated with heart failure, including myofibroblast proliferation, inflammation and fibrogenesis, tissue repair, and ventricular and tissue remodeling (Liu 2009).
Other Chronic Disease Preclinical evidence suggests that elevated galectin-3 levels may be linked to an array of other chronic diseases, including autoimmune disease. Levels of circulating galectin-3 have been associated with active disease in patient’s with Behcet’s disease (Ozden 2011), juvenille arthritis (Ezzat 2011), and inflammatory bowel disease (Frolova 2009); while increased local levels have been documented in patients with liver disease (Honsawek 2011, Wanninger 2011) and asthma/ chronic obstructive pulmonary disease (Pilette 2007). Preliminary in vivo studies have found that achieving reductions in circulating galactin-3 levels delivers therapeutic benefit for the diseases in question: arthritis (Forsman 2011, Wang 2010A), inflammatory gastrointestinal conditions (Fowler 2006, Srikanta 2010), hepatic disease (Honsawek 2011, Iacobini 2011), and asthma (Zuberi 2004).
FDA Approved Galectin-3 Serum Assay The direct link between galectin-3 and numerous acute and chronic disease states gives this novel molecule an important role in diagnostics and cardiovascular therapeutics, and this is becoming more widely accepted among the medical community, especially with the advent of a galectin-3 serum assay that can now accurately measure this “active” biomarker.
This inexpensive blood test can also be used by practitioners to assess cancer risk and progression, cirrhosis of the liver, kidney fibrosis, and other inflammation/fibrosis related conditions for which early detection is critical for optimal clinical outcome. Approximately 20% of patients exhibit changes in their galectin-3 levels every three months, and current evidence supports checking glactin-3 levels twice annually in those with stable disease (McCullough 2011). Furthermore, according to McCullough “doubling in galectin-3 level over the course of 6 months, irrespective of baseline value, identifies a high-risk patient in whom additional care … could be warranted” (2011). (See Figure 1 below outlining the reference ranges for normal and elevated serum galectin-3 in relation to disease risk).
Unlike the “bystander” biomarker C-reactive protein (CRP) that only indicates the presence of inflammation, elevated circulating galectin-3 is recognized as an “active” or “culprit” biomarker, since research shows that it actively mediates progression of numerous chronic illnesses. This means that galectin-3 is also a potentially important therapeutic target.
Targeting Galectin-3 with Modified Citrus Pectin
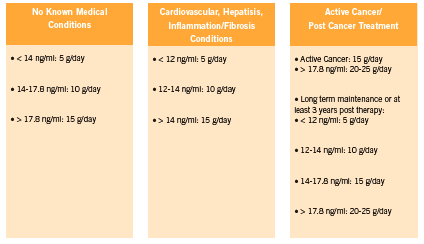
Although there are strong associations between galectin-3 levels and heart failure prognosis, and an impressive body of preclinical research linking galectin-3 levels to a variety of diseases, at present there is a lack of human interventional data on therapies that can lower galectin-3. There is preliminary but promising data coming out of preclinical studies however, with respect to modified citrus pectin for galectin-3 inhibition (Kidd 1996, Nangia-Makker 2002B, Olano-Martin 2003). (See Figure 2 for recommended dosages).
MCP is a form of citrus pectin that has been modified to a specific molecular weight and structure which allows it to be absorbed into the circulation and lends it its specific therapeutic properties. MCP is a complex polysaccharide fiber of repeating galacturonic acid groups with neutral sugar side chains. Regular (unmodified) citrus pectin derived from the pith of citrus fruit has a molecular weight of about 50-300 kiloDaltons, and a degree of esterification over 70%. These characteristics make regular pectin far too large to enter the blood stream. MCP can easily enter the circulation, however, when modified through a specific enzymatic process to achieve a molecular weight of 3-13 kiloDaltons, a degree of esterification under 10%, and its present specific structure (Eliaz 2006, Zhao 2008, Pieters 2006, Kidd 1996). Since galectin-3 is a beta-galactoside binding protein, and MCP is rich in beta-galactose, MCP has the ability to bind galectin-3, blocking galectin-3’s harmful effects (Nangia- Makker 2002A).
MCP is the only natural galectin-3 inhibitor demonstrated in published research that can modify the expression of galectin-3 through the natural galectin-binding affinity of its specific molecular structure, based on these authors’ literature review. For instance, a study in prostate cancer cells found that Gal-3 inhibition by antagonist GCS-100, a modified citrus pectin (MCP) product, increased cisplatin-induced apoptosis of PC3 cells (Wang 2010B). A mouse study found that MCP reduced colon cancer metastases to the liver (Liu 2008). In an animal model of an endothelial cancer, modified citrus pectin (MCP) caused a dose-dependent reduction in cancer cell survival by blocking galectin-3’s anti-apoptotic function (Johnson 2007). Furthermore, MCP sensitized cancer cells to doxorubicin such that the in vitro IC(50) of doxorubicin was reduced by 10.7-fold (Johnson 2007). Another study using breast and colon cancer xenografts models (transplanting cancer cell lines into animals and assessing growth) found that MCP was able to reduce growth, angiogenesis, and metastasis (Nangia- Makker 2002B). Another study found that MCP was able to decrease the adhesion of cancer cells to matrix (Inohara 1994). One study found that MCP can induce activation of NK cells in a leukemia model (Ramachandran 2011). Yan found that MCP has antiproliferative effects in prostate cancer cells (2010). In addition, by inhibiting galectin-3’s anti-apoptotic function and enhancing apoptosis induced by cytotoxic drugs, MCP holds the potential to dramatically increase the efficacy of conventional chemotherapy (Najmeh 2012), as well as natural and botanical compounds. These synergistic effects have been demonstrated in preclinical studies (Johnson 2007, Wang 2010B) and warrant further investigation in human clinical trials.
MCP: Beyond galectin-3
Two uncontrolled studies have examined the effect of MCP in cancer patients (Azemar 2007, Guess 2003). One study found a significantly higher PSA doubling time while on MCP for one year, compared to baseline in seven of 10 subjects (Guess 2003), while Azemar found that MCP increased ratings of quality of life overall after eight weeks (2007). Anti cancer effects were variable, with 11 of 29 assessable patients rated as having stable disease after 8 weeks (2 cycles), while 15 had progressive disease. Side effects included mild GI upset (flatulence, dyspepsia) and pruritis.
MCP has been shown to possess chelating activity in humans. As reported previously in IHP, MCP is a safe and potentially effective chelator of heavy metals and radioactive particles (Gallant 2010). MCP does not deplete essential minerals as other chelation therapies often do, and four uncontrolled trials show that patients reduced their toxic metal load by up to 76% (Eliaz 2006, Zhao 2008). MCP was dosed as 5g three times daily for three to six months.
Conclusion
As this important body of research continues to expand, galectin-3 testing is expected to become an integral component of cardiovascular and other screening panels — as routine as assessing cholesterol levels. There are a number of major laboratories that currently offer galectin-3 testing and with this simple assay, practitioners can gain more accurate insight into the risk, progression and advancement of numerous chronic inflammatory diseases. With the emergence of further research, galectin-3 is poised to become an important marker of cancer prognosis. Conversely, we can observe our patients experiencing significant clinical improvements, through firstline lifestyle modifications as well as through use of agents that can reduce inflammation and expression of galectin-3 – key among them, use of MCP.
References
Azemar M, Hildenbrand B, Haering B, Heim ME, Unger C. Clinical benefit in patients with advanced solid tumors treated with modified citrus pectin: a prospective pilot study. Clin Med: Oncol 2007, 1:73-80
Barrow H, Guo X, Wandall HH, Pedersen JW, Fu B, Zhao Q, Chen C, Rhodes JM, Yu LG. Serum galectin-2, -4, and -8 are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium. Clin Cancer Res. 2011 Nov 15;17(22):7035-46.
BG Medicine Inc. BGM Galectin-3 (Galectin-3 Assay). Document: LAB-IVD-001 R07. March 2011.
Brown ER, Doig T, Anderson N, Brenn T, Doherty V, Xu Y, Bartlett JM, Smyth JF, Melton DW. Association of galectin-3 expression with melanoma progression and prognosis. Eur J Cancer. 2012 Apr;48(6):865-74.
Chiu CG, Strugnell SS, Griffith OL, Jones SJ, Gown AM, Walker B, Nabi IR, Wiseman SM. Diagnostic utility of galectin-3 in thyroid cancer. Am J Pathol. 2010 May;176(5):2067-81.
deFilippi , CR, Felker, GM. Galectin-3 in Heart Failure—Linking Fibrosis, Remodeling, and Progression. U.S. Cardiology. 2010;7;1: 3–6.
de Boer RA, van Veldhuisen DJ, Gansevoort RT, Muller Kobold AC, van Gilst WH, Hillege HL, Bakker SJ, van der Harst P. The fibrosis marker galectin-3 and outcome in the general population. J Intern Med. 2012 Jul;272(1):55-64.
De Boer RA. Galectin-3 Levels & Mortality from All Causes in the General Population: PREVEND The Prevention of Renal and Vascular End-stage Disease (PREVEND) study results presented at the European Society of Cardiology (ESC) Congress (Aug) 2011a, in Paris, France. “Galectin-3, Cardiovascular Risk Factors and Outcome in the General Population.”
De Boer RA, Lok DJ, Jaarsma T, van der Meer P, Voors AA, Hillege HL, et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011b;43;1:60-8.
De Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail. 2009;11:811–7. 37.
Weigert J, Neumeier M, Wanninger J, Bauer S, Farkas S, Scherer MN, et al. Serum galectin-3 is elevated in obesity and negatively correlates with glycosylated hemoglobin in type 2 diabetes. J Clin Endocrinol Metab. 2010;95;3:1404-11.
Eliaz I, Hotchkiss A, Fishman M, Rode D. The effect of modified citrus pectin on urinary excretion of toxic elements. Phytother Res 2006, 20:859-864.
Ezzat MH, El-Gammasy TM, Shaheen KY, Osman AO. Elevated production of galectin-3 is correlated with juvenile idiopathic arthritis disease activity, severity, and progression. Int J Rheum Dis. 2011 Oct;14(4):345-52.
Felker GM, Fiuzat M, Shaw LK, Clare R, Whellan DJ, Bettari L, Shirolkar SC, Donahue M, Kitzman DW, Zannad F, Piña IL, O’Connor CM. Galectin-3 in ambulatory patients with heart failure: results from the HF-ACTION study. Circ Heart Fail. 2012 Jan 1;5(1):72-8.
FDA, Food and Drug Administration. Galectin-3 in vitro diagnostic assay. Updated 17 June 2012. URL http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/ classification.cfm?ID=627 Accessed 20 June 2012.
Forsman H, Islander U, Andréasson E, Andersson A, Onnheim K, Karlström A, et al. Galectin 3 aggravates joint inflammation and destruction in antigen-induced arthritis. Arthritis Rheum. 2011;63;2:445-54.
Fowler M, Thomas RJ, Atherton J, Roberts IS, High NJ. Galectin-3 binds to Helicobacter pylori O-antigen: it is upregulated and rapidly secreted by gastric epithelial cells in response to H. pylori adhesion. Cell Microbiol. 2006;8;1:44-54.
Frol’ová L, Smetana K Jr, Borovská D, Kitanovicová A, Klimesová K, Janatková I, Malícková K, Lukás M, Drastich P, Benes Z, Tucková L, Manning JC, André S, Gabius HJ, Tlaskalová-Hogenová H. Detection of galectin-3 in patients with inflammatory bowel diseases: new serum marker of active forms of IBD? Inflamm Res. 2009 Aug;58(8):503-12.
Gallant J, Rouchotas P. Modified citrus pectin (MCP): heavy metal chelator, possible cancer treatment. IHP Sept 2010: 58-61.
Guess BW, Scholz MC, Strum SB, Lam RY, Johnson HJ, Jennrich RI. Modified citrus pectin (MCP) increases the prostate-specific antigen doubling time in men with prostate cancer: a phase II pilot study. Prostate Cancer Prostatic Dis 2003;6:301-304.
Gullestad L, Ueland T, Kjekshus J, Nymo SH, Hulthe J, Muntendam P, Adourian A, Böhm M, van Veldhuisen DJ, Komajda M, Cleland JG, Wikstrand J, McMurray JJ, Aukrust P; on behalf of the CORONA Study Group. Galectin-3 predicts response to statin therapy in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA). Eur Heart J. 2012 Apr 26.
Honjo Y, Nangia-Makker P, Inohara H, Raz A. Down-regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells. Clin Cancer Res. 2001 Mar;7(3):661-8.
Honsawek S, Chongsrisawat V, Praianantathavorn K, Theamboonlers A, Poovorawan Y. Elevation of serum galectin-3 and liver stiffness measured by transient elastography in biliary atresia. Eur J Pediatr Surg. 2011;21;4:250-4.
Iacobini C, Menini S, Ricci C, Fantauzzi CB, Scipioni A, Salvi L, et al. Galectin-3 ablation protects mice from diet-induced NASH: a major scavenging role for galectin-3 in liver. J Hepatol. 201;54;5:975-83.
Inohara H, Raz A. Effects of natural complex carbohydrate (citrus pectin) on murine melanoma cell properties related to galectin-3 functions. Glycoconj J. 1994 Dec;11(6):527-32.
Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 2000 Apr;6(4):1389-93
Johnson KD, Glinskii OV, Mossine VV, Turk JR, Mawhinney TP, Anthony DC, Henry CJ, Huxley VH, Glinsky GV, Pienta KJ, Raz A, Glinsky VV. Galectin-3 as a potential therapeutic target in tumors arising from malignant endothelia. Neoplasia. 2007 Aug;9(8):662-70.
Kidd P. A new approach to metastasis cancer prevention: modified citrus pectin (MCP), a unique pectin that blocks cell surface lectins. Altern Med Rev 1996;1:4-10.
Lin YH, Lin LY, Wu YW, Chien KL, Lee CM, Hsu RB, Chao CL, Wang SS, Hsein YC, Liao LC, Ho YL, Chen MF. The relationship between serum galectin-3 and serum markers of cardiac extracellular matrix turnover in heart failure patients. Clin Chim Acta. 2009 Nov;409(1-2):96-9.
Liu HY, Huang ZL, Yang GH, Lu WQ, Yu NR. Inhibitory effect of modified citrus pectin on liver metastases in a mouse colon cancer model. World J Gastroenterol 2008, 14:7386-7391.
Liu YH, D’Ambrosio M, Liao TD, Peng H, Rhaleb NE, Sharma U, André S, Gabius HJ, Carretero OA. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol. 2009 Feb;296(2):H404-12.
Lok DJ, Van Der Meer P, de la Porte PW, Lipsic E, Van Wijngaarden J, Hillege HL, et al. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol. 2010;99;5:323-8.
McCullough PA, Olobatoke A, Vanhecke TE. Galectin-3: a novel blood test for the evaluation and management of patients with heart failure. Rev Cardiovasc Med. 2011;12(4):200-10.
Najmeh T, Houri S, Parvin M, Firouzeh B, Arash HN, Abdolfattah S, Ebrahim H. Combination effect of PectaSol and Doxorubicin on viability, cell cycle arrest and apoptosis in DU-145 and LNCaP prostate cancer cell lines. Cell Biology International 2012, Immediate Publication, doi:10.1042/CBI20110309.
Nangia-Makker P, Conklin J, Hogan V, Raz A. Carbohydrate-binding proteins in cancer, and their ligands as therapeutic agents. Trends Mol Med 2002; 8:187-192.A
Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, et al. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Ins 2002;94:1854-1862.B
Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ, et al. Galectin-3 induces endothelial cell morphogenesis and angiogensis. Am J Pathol 2000, 156:899-909.
Olano-Martin E, Rimbach GH, Gibson GR, Rastall RA. Pectin and pecticoligosaccharides induce apoptosis in in vitro human colonic adenocarcinoma cells. Anticancer Res 2003;23:341 346.
Özden MG, Caycı YT, Tekin H, Çoban AY, Aydın F, Sentürk N, Bek Y, Cantürk T, Turanlı AY. Serum galectin-3 levels in patients with Behçet’s disease: association with disease activity over a long-term follow-up. J Eur Acad Dermatol Venereol. 2011 Oct;25(10):1168-73.
Peng W, Wang HY, Miyahara Y, Peng G, Wang RF. Tumor-associated galectin-3 modulates the function of tumor-reactive T cells. Cancer Res. 2008 Sep 1;68(17):7228-36.
Pienta KJ, Naik H, Akhtar A, Yamazaki K, Replogle TS, Lehr J, et al. Inhibition of spontaneous metastasis in a rat prostate cancer model by oral administration of modified citrus pectin. J Natl Cancer Inst 1995;87:348-353.
Pieters RJ. Inhibition and detection of galectins. Chembiochem. 2006 May;7(5):721-8.
Pilette C, Colinet B, Kiss R, André S, Kaltner H, Gabius HJ, Delos M, Vaerman JP, Decramer M, Sibille Y. Increased galectin-3 expression and intra-epithelial neutrophils in small airways in severe COPD. Eur Respir J. 2007 May;29(5):914-22.
Ramachandran C, Wilk BJ, Hotchkiss A, Chau H, Eliaz, I Melnick SJ. Activation of human T-helper/inducer cell, T-cytotoxic cell, B-cell, and natural killer (NK)-cells and induction of natural killer cell activity against K562 chronic myeloid leukemia cells with modified citrus pectin. BMC Complementary and Alternative Medicine. 2011;11:59.
Saleh HA, Feng J, Tabassum F, Al-Zohaili O, Husain M, Giorgadze T. Differential expression of galectin-3, CK19, HBME1, and Ret oncoprotein in the diagnosis of thyroid neoplasms by fine needle aspiration biopsy. Cytojournal. 2009 Sep 18;6:18.
Saussez S, Lorfevre F, Lequeux T, Laurent G, Chantrain G, Vertongen F, Toubeau G, Decaestecker C, Kiss R. The determination of the levels of circulating galectin-1 and -3 in HNSCC patients could be used to monitor tumor progression and/or responses to therapy. Oral Oncol. 2008 Jan;44(1):86-93.
Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12;8:826-32.
Srikanta BM, Sathisha UV, Dharmesh SM. Alterations of matrix metalloproteinases, gastric mucin and prostaglandin E(2) levels by pectic polysaccharide of swallow root (Decalepis hamiltonii) during ulcer healing. Biochimie. 2010;92;2:194-203.
Tinari N, Kuwabara I, Huflejt ME, Shen PF, Iacobelli S, Liu FT. Glycoprotein 90K/ MAC-2BP interacts with galectin-1 and mediates galectin-1-induced cell aggregation. Int J Cancer. 2001 Jan 15;91(2):167-72.
Wang YG, Kim SJ, Baek JH, Lee HW, Jeong SY, Chun KH. Galectin-3 Increases the Motility of Mouse Melanoma Cells by Regulating MMP-1 Expression. Exp Mol Med. 2012 Mar 21. [Epub ahead of print]
Wang CR, Shiau AL, Chen SY, Cheng ZS, Li YT, Lee CH, et al. Intra-articular lentivirus-mediated delivery of galectin-3 shRNA and galectin-1 gene ameliorates collagen-induced arthritis. Gene Ther. 2010;17;10:1225-33.A
Wang Y, Nangia-Makker P, Balan V, Hogan V, Raz A. Calpain activation through galectin-3 inhibition sensitizes prostate cancer cells to cisplatin treatment. Cell Death Dis. 2010 Nov 18;1:e101.B
Wang Y, Nangia-Makker P, Tait L, Balan V, Hogan V, Pienta KJ, et al. Regulation of prostate cancer progression by galectin-3. Am J Pathol. 2009;174;4:1515-23.
Wanninger J, Weigert J, Wiest R, Bauer S, Karrasch T, Farkas S, Scherer MN, Walter R, Weiss TS, Hellerbrand C, Neumeier M, Schäffler A, Buechler C. Systemic and hepatic vein galectin-3 are increased in patients with alcoholic liver cirrhosis and negatively correlate with liver function. Cytokine. 2011 Sep;55(3):435-40.
Weigert J, Neumeier M, Wanninger J, Bauer S, Farkas S, Scherer MN, et al. Serum galectin-3 is elevated in obesity and negatively correlates with glycosylated hemoglobin in type 2 diabetes. J Clin Endocrinol Metab. 2010;95;3:1404-11.
Yan J, Katz A. PectaSol-C modified citrus pectin induces apoptosis and inhibition of proliferation in human and mouse androgen-dependent and- independent prostate cancer cells. Integr Cancer Ther 2010;9:197-203.
Yu LG, Andrews N, Zhao Q, McKean D, Williams JF, Connor LJ, et al. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancerassociated MUC1 causes increased cancer cell endothelial adhesion. J Biol Chem. 2007;5;282(1):773-81.
Yu LG. Circulating galectin-3 in the bloodstream: An emerging promoter of cancer metastasis. World J Gastrointest Oncol. 2010 Apr 15;2(4):177-80.
Zaia Povegliano L, Oshima CT, de Oliveira Lima F, Andrade Scherholz PL, Manoukian Forones N. Immunoexpression of galectin-3 in colorectal cancer and its relationship with survival. J Gastrointest Cancer. 2011 Dec;42(4):217-21.
Zhao ZY, Liang L, Fan X, Yu Z, Hotchkiss AT, Wilk BJ, et al. The role of modified citrus pectin as an effective chelator of lead in children hospitalized with toxic lead levels. Altern Ther Health Med 2008, 14:34-38.
Zhao Q, Guo X, Nash GB, Stone PC, Hilkens J, Rhodes JM, et al. Circulating galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface. Cancer Res. 2009;69;17:6799-806.
Zuberi RI, Hsu DK, Kalayci O, Chen HY, Sheldon HK, Yu L, et al. Critical role for galectin-3 in airway inflammation and bronchial hyperresponsiveness in a murine model of asthma. Am J Pathol. 2004;165;6:2045-53.









