Low Dose Naltrexone
Role as adjunct cancer treatment
Abstract
Research in the area of naltrexone has shown both promise and controversy for cancer treatment. Ongoing validation regarding the differing mechanisms of action, the dosage, and the timing of the dose is required. In reviewing the mechanism of action for low-dose naltrexone, more were identified than originally anticipated, which range from a vague endogenous immune response to a complex mechanisms involving LDN affecting a blockade at the OGF-OGFr axis. This article presents the historical use of naltrexone, the current information about the low-dose options and use recommendations, the receptors involved with some controversial effects on cancer with respect to mechanisms of action, and calls for more evidence in the form of phase three clinical trials.
Naltrexone – Mechanism of Action in Treatment of Cancer
The task of bringing together single dimension information from laboratory research examining cellular, vascular, and endocrine events to understand the three dimensional mechanisms of disease in the human body is daunting. The reliance upon research and scientific principles weighs heavy in cancer where much is unknown about disease progression, especially in the form of metastasis or recurrence. Currently information is elucidated about mechanisms of action, from which arise more questions and theories, which in turn provokes the critically-thinking reader to utilize the available information to the best of their abilities. An example of the evolution of a treatment that was first presented as novel and which is now entering the realm of evidence-based medicine is that of low dose Naltrexone (LDN) for use in autoimmune disease and cancer. For the purpose of this article, LDN and its effects in cancer will be the primary focus.
There is little clinical or research evidence beyond the level of dramatic reports from Dr.’s Bihari, Berkson and Rubin (Berkson 2009) to support the use of LDN in treatment of cancer. The authors present case reports of three people diagnosed with pancreatic cancer, treated successfully with the use of Alpha Lipoic Acid and LDN, and call for clinical trials for further investigation of their protocol. In elucidating the mechanism of action for this success they suggested an endogenous immune response. Suffice to say it is a start, and a proposed mechanism of action may eventually create further research to prove or disprove. Further, there is recent evidence in cell studies elucidating more than one anti-cancer mechanism of LDN. Low-dose Naltrexone (LDN) presents itself with complexity: there are different receptors involved, and the resulting effects of LDN on cancer have varied widely. This article reviews the recent data regarding the mechanisms of action and the proposed resulting effects of LDN in treatment of cancer.
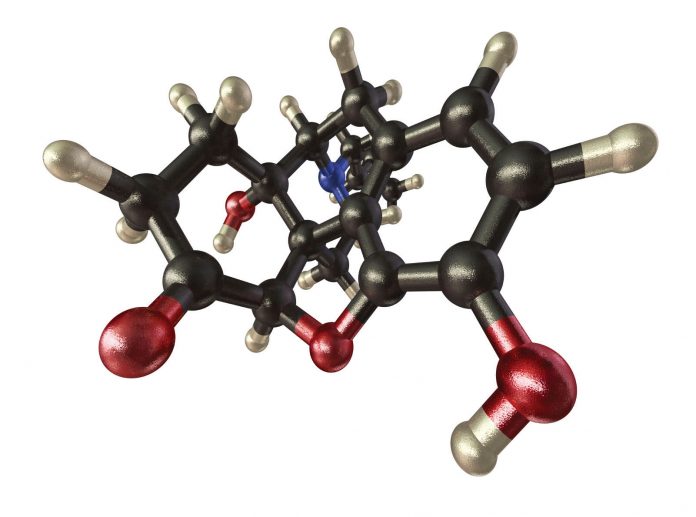
Naltrexone History in Brief Naltrexone is used to treat opioid substance abuse and acts by blocking opioid receptors in the brain. It was approved by the US FDA in 1984 in 50 mg dose increments as a competitive narcotic antagonist, for the purpose of stopping or reducing the effects of a heroin or opium overdose and/or for the reduction of opioid withdrawal symptoms (Lowdosenaltrexone.org May 4 2010). For the purposes of opioid blockade this 50mg ‘high dose’ Naltrexone is in tablet form for oral consumption away from meals. Depending upon the type of addiction there are different prescription regimens ranging from daily to every other day schedules (PubMed Health 2011). There is very little understood about Naltrexone beyond opioid blockade as the patent expiry led to it becoming dormant as a prescription item for a number of years. For the treatment of addiction, ‘high-dose’ Naltrexone exerts opposite effects on the Mu Opioid Protein (MOP) receptor at the plasma membrane, and can inhibit secretion of opioids in both brain and adrenal glands via beta endorphins and enkephalins (Figure 1). Naltrexone has been used to treat opioid overdose, opioid addiction (Gonzalez 2004) and addictions to other substances such as alcohol (Chick 2000, Davidson 1999). Naltrexone is conventionally dosed up to a maximum of 350 mg QD. This high-dose use is limited by the adverse effects which are similar to opioid withdrawal and these effects can even occur in patients without previous exposure to opioids (Hollister 1981).
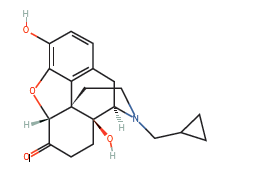
In contrast the Low-dose naltrexone (LDN) dose ranges from 0.1-4.5 mg QD and has not produced adverse effects. The off-label use of low-dose Naltrexone started with Dr. Bihari and has been in use since the 1980’s, subsequently described via case report as an anti-cancer strategy in 2009 by Berkson and Rubin. LDN has been used off-patent for treatment of autoimmune illnesses and cancer since the late 1980’s. It is compounded into tablets or a powder which is mixed with water for oral consumption. Comparative absorption data and information on suppository or injectable doses is limited and unreliable at this time. LDN interacts with opioid analgesics such as Hydrocodone, Oxycodone, Oxymorphone and other opiate/opioid narcotics and thus should be avoided in such instances. It is recommended to gradually reduce the intake and then eliminate the use of any opioid narcotic for 10-12 days before starting LDN. The compounded powder for oral consumption of LDN has a shorter, temperature-sensitive shelf-life and a bitter taste when taken on its own, in comparison to the compounded LDN tablet for oral consumption (Figure 2) (Fawcett 1997).
Receptor Review
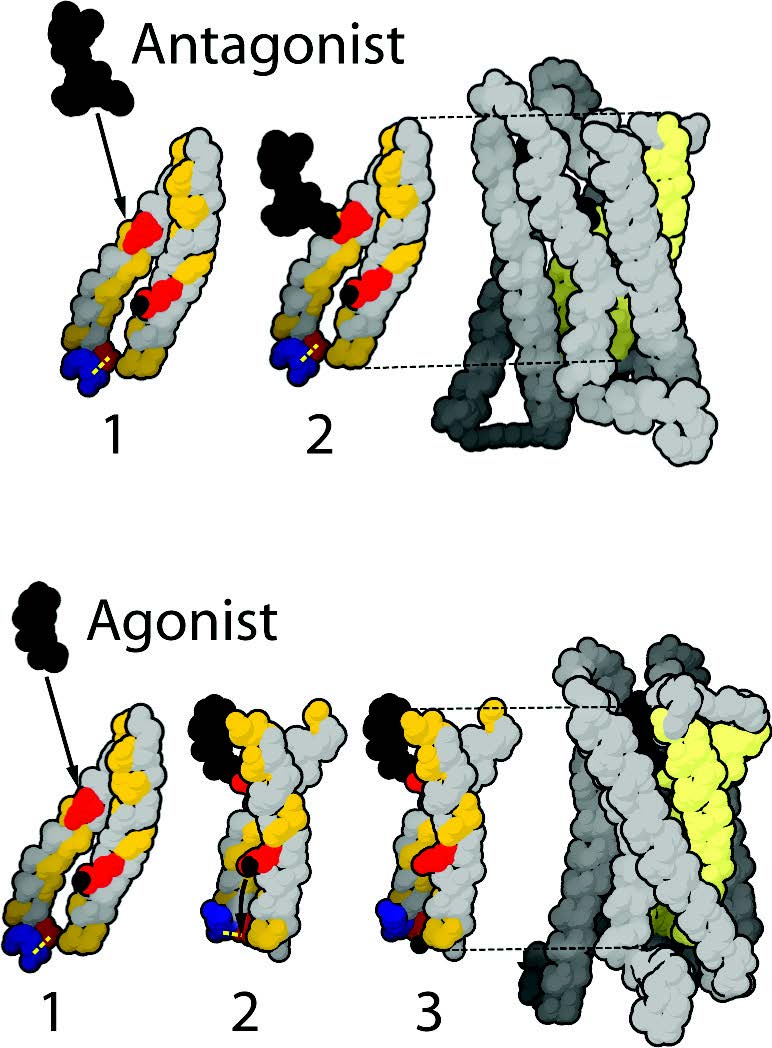
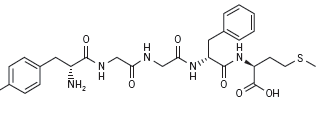
Transmembrane receptors in the human body regulate the majority of cellular metabolism and physiology. The largest group of receptors belongs to a group coupled to G-proteins (G-Protein-Coupled Receptors, or GPCRs) (Lefkowitz 2007). The superfamily of GPCRs regulates metabolism, including cellular replication; they are the targets for drug therapy in treatment of cancer and autoimmune disease (Jacoby 2006, Pierce 2002). The genes encoding μ-, δ-, κ-, ζ- and nociceptin Opioid Proteins (MOP- Figure 1, DOP, KOP, OGFr, NOP respectively) belong in the super-family of the GPCRs (Brown 2008, Corbett 2006, Wang 2001) are the transmembrane receptors targeted in the treatment of narcotic abuse (Wang 2001), and are suggested to be the target in the treatment of autoimmune disease (Zagon 2009) and cancer with LDN (Brown 2008). However, more recent research has indicated that the regulatory effect on cancer cell growth extends beyond that of the MOP and to an endogenous opioid factor also referred to as Met5-Enkephalin or Opioid Growth Factor (OGF, Figure 3), and its receptor the ζ-opioid receptor or Opioid Growth Factor Receptor (OGFr). OGF has a role in growth inhibition and OGFr determines the properties of cell proliferation (Zagon 2003). Ongoing phase one and two trials on squamous cell carcinoma of the head and neck and hepatocellular cancer respectively are investigating how OGF binds and activates the OGFr to inhibit angiogenesis and tumor cell growth (Clinicaltrials.gov 2010A, Clinicaltrials.gov 2010B).
Naltrexone – Past, Present and Future
Naltrexone (Figure 2) was approved by the US FDA in 1984 in 50mg doses (max dose 350mg) and acts as a narcotic antagonist. It is used to treat and stop heroin or opium overdoses and withdrawal (Gonzalez 2004), and to treat substance addiction such as alcoholism (Chick 2000, Davidson 1999). Since the patent expired other uses have thus been investigated. In the 1990’s Dr. Bihari first used LDN to treat people with AIDS and then later on to treat people with cancer (Bihari 1995, Lowdosenaltrexone.
org May 18 2010). There have been reports of successful treatment of various cancers (Berkson 2009, Dagleish 2005, Hytrek 1996, Zagon 2000), AIDS (Bihari 1995, Gekker 2001), Multiple Sclerosis (Cree 2010), Autism (Panskepp 1991), and autoimmune disorders such as Crohn’s Disease (Smith 2007) and fibromyalgia (Brown 2008). Sustained release naltrexone (32mg) has now completed investigation in Phase 2 trials with bupropion for the treatment of obesity (Clinicaltrials.gov 2010B).
Naltrexone – Mechanisms of Action
As an opioid antagonist, high dose (50mg – 350mg) naltrexone blocks secretion of opioids in the brain and adrenal glands via the MOP. At low dose (3-4.5mg) nocturnal dose-timing of naltrexone is hypothesized to block opioid receptor expression (MOP, DOP, KOP) and increases circulation of met-enkephalin (Figure 3) and beta-endorphin for up to six hours. The MOP is hypothesized to play a role in the various responses of the immune system to stress, infection and malignant transformation (Makman 1994). LDN has several proposed theoretical mechanisms of action depending on whether the treatment is targeted for cancer or autoimmune disease. An recent cell study investigation revealed controversy. In the anti-cancer role of LDN the OGF/OGFr axis can be allosterically modulated to create multi-dimensional effects on signaling pathways for cellular replication. Allosteric modulation is also demonstrated by the human immune system and readers will recall that the subtypes of Th cells are allosterically modulated to create immune functionality. According to the investigator Dr. Ian Zagon at Penn State University (e-mail communication May 12, 2010): “All human cancers are equipped with the OGF/OGFr axis and depend on it for regulation of cell proliferation. NTX (naltrexone) works by blocking the OGF-OGFr axis. It causes an up-regulation. If the dose is low enough, you allow time for OGF to interact with OGFr to cause a depression in cell proliferation. If you give repeated low doses or a high dose you get up-regulation but no interaction between peptide and receptor. Since the OGF-OGFr axis is tonically active – it is on all the time and watching over the pace of cell proliferation by inhibitory pathways, blockade of OGF-OGFr by naltrexone continuously allows cells to be unregulated in cell division and you have more cells produced.” For the purpose of illustrating further the complexity of this drug there are several mechanisms of action proposed in treatment of the following different types of cancer:
•In neuroblastoma, the low (3-4.5mg) and high dose (100 mg) naltrexone administered demonstrated a direct modulating effect on oncogenesis. The low dose naltrexone elevated opiate receptors and circulating beta endorphin and met-enkephalin through the four to six hour receptor blockade (McLaughlin 1987) and resulted in decreased oncogenesis.
• A small study of 14 patients with untreatable metastatic solid tumors treated with melatonin (20mg hs) and naltrexone(100mgQD) to augment Interleukin-2 therapy demonstrated that lymphocyte production is amplified compared with melatonin and IL-2 therapy alone (Lissoni 2002).
• In Nude mice with colon cancer (HT-29) treated with 0.1mg/ kg naltrexone demonstrated that tumorigenicity was inhibited by opioids via a 2.5 increase in plasma met5-enkephalin in naltrexone-treated mice and an 85% reduction of binding capacity of 3H-Met5-enkephalin in tumor tissue when compared to the control group (Hytrek 1996).
• In pancreatic cancer MIA PaCa-2 clonal cell lines the singlelow- dose naltrexone-induced amplification of the OGFr axis delayed the G1/S inter-phase of the cell cycle via CDK inhibition of p16 or p21, causing a decrease in CDK4/CDK2 activity and RB protein phosphorylation, thus halting DNA synthesis and subsequent cell growth (Zagon 2007).
It remains to be determined how LDN exerts its effects via either an independent or combined extracellular or cytoplasmic effect on cancer cell replication. These give rise to the confirmation that often LDN is not a single therapy provided in isolation of other cancer treatment, and that there are pharmacokinetic properties to LDN which affect enkephalin production, lymphocyte production, and cyclin dependent kinases on control of the cell cycle.
Summary The number of cancer survivors alive compared to those seeking a cure can be described as an inverse relationship. There is a distinct lack of cancer survivors, in spite of all the enormity of efforts ongoing to seek a cure. Research in the area of naltrexone has shown both promise and controversy for cancer treatment and requires ongoing validation regarding the differing mechanisms of action, the dosage, and the timing of the dose and the type of cancer. In reviewing the mechanism of action for low-dose naltrexone, more were identified than originally elucidated by historical data and case reports. An endogenous immune response has been suggested via case report as one possible theory, as well as an increase in enkephalins, lymphocytes, and allosteric blockade at the OGF-OGFr axis. It is a daily challenge to understand the intended and untoward influences of naltrexone upon tumor cell growth because of the differing receptors involved and to tailor a specific protocol based upon the current evidence for people with cancer. In looking to the future use of LDN, there is a need for collaborative effort between the research bench and the patient that may provide more information regarding the mechanisms of tumor initiation, progression and promotion to help extend the limit of one’s knowledge, skills and judgment when treating the complex disease of cancer.
References
Berkson BM, Rubin DM, Berkson AJ. Revisiting the ALA/N (alpha-lipoic acid/lowdose naltrexone) protocol for people with metastatic and nonmetastatic pancreatic cancer: a report of 3 new cases. Integr Cancer Ther. 2009 Dec;8(4):416-22.
Bihari B. Efficacy of low dose naltrexone as an immune stabilizing agent for the treatment of HIV/AIDS [letter]. AIDS Patient Care. 1995 Feb;9(1):3.
Brown N, Panskepp J. Low-dose naltrexone for disease prevention and quality of life. Med Hypotheses. 2008;72(3):333-7.
Chick J, Anton R, Checinski K, Croop R, Drummond DC, Farmer R, Labriola D, Marshall J, Moncrieff J, Morgan MY, Peters T, Ritson B. A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcohol. 2000 Nov-Dec;35(6):587-93.
Clinicaltrials.gov. http://www.cancer.gov / clinicaltrials /search /view?cdrid = 599953&ver sion =HealthProfessional & protocolsearchid = 8291854. Accessed Nov 2010A.
Clinicaltrials.gov. http://clinicaltrials.gov/ct2/show/NCT00711477. Accessed Nov 2010B.
Corbett AD, Henderson G, McKnight AT, Paterson SJ. 75 years of opioid research: the exciting but vain quest for the Holy Grail. Br J Pharmacol. 2006 Jan;147 Suppl 1:S153-62.
Cree BA, Kornyeyeva E, Goodin DS. Pilot trial of low-dose naltrexone and quality of life in multiple sclerosis. Ann Neurol. 2010 Aug;68(2):145-50.
Dalgleish A, Whelan M. Novel immunotherapeutic approaches to prostate cancer. Curr Opin Mol Ther. 2005 Feb;7(1):30-4.
Davidson D, Palfai T, Bird C, Swift R. Effects of naltrexone on alcohol selfadministration in heavy drinkers. Alcohol Clin Exp Res. 1999 Feb;23(2):195-203.
Fawcett JP, Morgan NC, Woods DJ. Formulation and stability of naltrexone oral liquid for rapid withdrawal from methadone. Ann Pharmacother. 1997 Nov;31(11):1291-5.
Gekker G, Lokensgard J, Peterson PK. Naltrexone potentiates anti-HIV-1 activity of antiretroviral drugs in CD4+ lymphocyte cultures. Drug Alc Depend. 2001;64:257-63.
Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Expert Opin Pharmacother. 2004 Apr;5(4):713-25.
Hollister LE, Johnson K, Boukhabza D, Gillespie HK. Aversive effects of naltrexone in subjects not dependent on opiates. Drug Alcohol Depend. 1981 Aug;8(1):37-41.
Hytrek SD, McLaughlin PJ, Lang CM, Zagon IS. Inhibition of human colon cancer by intermittent opioid blockade with naltrexone. Cancer Lett. Mar 29, 1996;101(2):159-64.
Jacoby E, Boulehal R, Gerspacher M, Seuwen K. The 7 TM G-protein-coupledreceptor target family. ChemMedChem. 2006;1(8):761-82.
Lefkowitz RJ. Seven transmembrane receptors: something old, something new. Acta Physiol (Oxf). 2007 May;190(1):9-19.
Lissoni P, Malugani F, Malysheva O, Kozlov V, Laudon M, Conti A, Maestroni G. Neuroimmunotherapy of untreatable metastatic solid tumors with subcutaneous lowdose interleukin-2, melatonin and naltrexone: modulation of interleukin-2-induced antitumor immunity by blocking the opioid system. Neuro Endocrinol Lett. 2002 Aug;23(4):341-4.
Lowdosenaltrexone.org. www.lowdosenaltrexone.org. Accessed May 4 2010.
Lowdosenaltrexone.org. www.lowdosenaltrexone.org/ldn_and_cancer.htm. Accessed May 18 2010.
Makman M. Morphine receptors in immunocytes and neurons. Adv Neuroimmunol. 1994;4:69-82.
McLauMghlin PJ, Zagon I. Modulation of human neuroblastoma transplanted into nude mice by endogenous opioid systems. Life Sci. 1987;41:1465-72.
Panksepp J, Lensing P, Leboyer M, Bouvard MP. Naltrexone and other potential new pharmacological treatments of autism. Brain Dysfunc. 1991;4:281-300.
Pierce KL, Premont R, Lefkowitz RJ. Seven transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639-50.
PubChem. http://pubchem.ncbi.nlm.nih.gov. Accessed Nov 2010.
PubMed Health. http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0000853/ accessed July 17 2011.
Smith J, Stock H, Bingaman S, Mauger D, Rogosnitzky M, Zagon I. Low-dose naltrexone therapy improves active Crohn’s disease. Amer J Gastroenterology. 2007;102(4):820-8.
Wang D, Rachal KM, Bilsky EJ, Sadee W. Inverse Agonists and neutral antagonists at mu opioid receptor (MOR): possible role of basal receptor signaling in narcotic dependence. J Neurochem. 2001;77(6):1590-600.
Zagon IS, McLaughlin P. Opioids and the apoptotic pathway in human cancer cells. Neuropeptides. 2003;37:79-88.
Zagon IS, Rahn KA, Turel KP, McLaughlin PJ. Endogenous Opioids Regulate Expression of Experimental Autoimmune Encephalomyelitis: A New Paradigm for the Treatment of Multiple Sclerosis. Exp Biol Med. 2009;234(11):1383-92.
Zagon IS, Roesener CD, Verderame MF, Ohlsson-Wilhelm BM, Levin RJ, McLaughlin PJ. Opioid growth factor regulates the cell cycle of human neoplasias. Int J Oncol. 2000;17:1053-61.
Zagon IS, Verderame MF, Hankins J, McLaughlin PJ. Overexpression of the opioid growth factor receptor potentiates growth inhibition in human pancreatic cancer cells. International Journal of Oncology. 2007;30(4):775-783.