Cancer induced cachexia (CIC)
Effective integrative management
Abstract
Cancer induced cachexia (CIC) is a syndrome of muscle wasting accompanied by anorexia with or without loss of adipose tissue. Underlying CIC is a complex interaction of proinflammatory cytokines that act centrally on the hypothalamus and peripherally to increase catabolism and resting energy expenditure, while decreasing protein synthesis. The presence of CIC is an important prognostic indicator in cancer patients. Nutritional strategies utilized by naturopathic doctors have been proven to slow down this condition, induce weight gain, and improve cancer related outcomes. Such therapies include high EPA fish oil, melatonin, L-carnitine, and branched chain amino acids and/ or whey protein.
Cancer induced cachexia (CIC) is a multifactorial syndrome characterized by loss of skeletal muscle, accompanied by anorexia and (sometimes) loss of adipose tissue (Fearon 2011). A number of underlying metabolic derangements are implicated in CIC, and associated symptoms may include malnutrition, anemia, fatigue, and decreased muscle strength & impaired physical function. Cachexia, defined as a loss of body weight greater than 5%, leads to poor performance, poor quality of life, increased complications, and higher mortality rate among cancer patients (Burckart 2010, Fearon 2011). Targeted nutritional interventions are an important part of cachexia prevention and management. This paper reviews several key agents that can improve outcomes in cachexia, among them fish derived omega-3 fatty acids (EPA and DHA), melatonin, branched chain amino acids (BCAAs), l-carnitine, and whey protein.
Cachexia is a therapeutic target of high importance in cancer patients due to its strong prognostic significance (Burckhart 2010). It is widely recognized that cachexia leads to poorer cancer outcomes, and is reported as the direct cause of death in up to 20-40% of cancer patients (Fox 2009). Recently, Yang et al found that, among a large cohort of lung cancer patients (n=14,751), those who showed weight loss (15.8% average) at time of diagnosis had significantly shorter survival time compared to those who did not, 6.4 versus 9.2 mo, P < 0.001 (2011). Similar results have been found for other cancers as well, including colorectal cancer and pancreatic cancer, with up to two fold greater increase in risk of death (HR = 2.26; CI 1.18-4.32; P = 0.014) reported (Bachmann 2008, Thoreson 2012). Some teams have used serum albumin as a marker of malnutrition and cachexia, and low levels have been associated with decreased survival in ovarian, breast, and other cancers (Asher 2011, Lis 2003, Polterauer 2010). Cancer related weight loss is associated with decreased tolerance to anticancer therapy, and significantly predicts toxicity from cancer treatment (Fearon 2011, Ross 2004). Naito found that cachexia is associated with altered oxycodone pharmacokinetics due to decreased albumin levels (an important drug binding and transporting protein), resulting in increased levels of free drug and increased incidence of central adverse reactions (2012). Despite this, cachexia remains under-recognized in patients, and under-treated as a clinical entity (Churm 2009, Spiro 2006).
The molecular mechanisms underlying CIC are still being elucidated, however, pro-inflammatory cytokines such as IL-1, IL-2, IL-6, interferon gamma and TNF α have a key role in mediating cancer cachexia (Macdonald 2003). IL-6 is a key cytokine in iniating multiple proinflammatory pathways including the acute phase response, and is known to be produced by the tumor microenvironment (Oshima 2012, Zamarron 2011). The inflammatory basis of CIC distinguishes it from age related sarcopenia or frank starvation (Pepersack 2011), and presents the therapeutic rationale for intervention with agents such as EPA, NSAIDS, and celecoxib, a COX-2 inhibitor. Ultimately, these pro-inflammatory cytokines initiate a cascade of events in the hypothalamus and peripherally that results in detrimental metabolic changes, including increased catabolism (proteolysis and lipolysis) and resting energy expenditure, reduced muscle protein synthesis, and anorexia (Burckart 2010). According to a more simple summation by Fearon, “The pathophysiology [of cachexia] is characterized by a negative protein and energy balance driven by a variable combination of reduced food intake and abnormal metabolism” (2011).
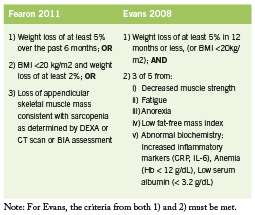
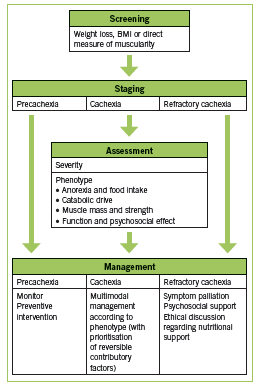
Various definitions of CIC have been used. Two recent sets of diagnostic criteria are outlined in Table 1. These criteria by Fearon are recently proposed in a consensus statement published in Lancet Oncology (2011), while those by Evans are older and incorporate biochemical parameters (2008). Recently, work has been done to establish staging of cachexia. Precachexia is defined as “weight loss ≤5%, with anorexia and metabolic changes” (Fearon 2011). Cachexia is as defined below (Fearon 2011). Refractory cachexia is variable in terms of severity, with the presence of procatabolic state; cancer not responsive to anticancer treatment; low performance score; and <3 months expected survival (Fearon 2011). A proposed algorithm in terms of assessment and management is adapted in Figure 1. (Valium)
Nutritional Interventions Fish Derived Omega-3 Fatty Acids
Fish oil is rich in the omega-3 polyunsaturated fatty acids eicosapentanoic acid (EPA) and docosahexanoic acid (DHA), and has been shown to suppress the production of proinflammatory cytokines in both healthy volunteers and cancer patients, leading to weight stabilization, improved immune function, and better cancer treatment outcomes (Barber 2001, Wigmore 2000). Evidence on use of EPA in various cancer types is summarized in Table 2.
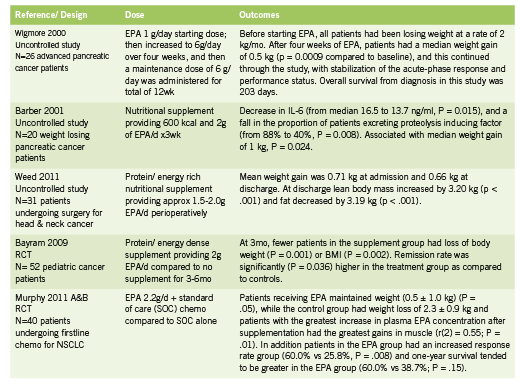
A 2007 systematic review found that oral supplements enriched with omega-3 fatty acids increased weight gain, increased appetite, improved quality of life and reduced post-surgical morbidity in patients with advanced pancreatic and upper gastrointestinal cancer (Colomer 2007). Not all trials have shown such promising effects (Fearon 2006), however, though the reason may have to do with cancer stage, study duration, and differences between cancer types and phenotypes. Although the dosages used in clinical trials shows some variability, a dose of at least 2g EPA per day is recommended. No serious side effects have been reported in the trials reviewed; minor side effects typically include fishy aftertaste and mild nausea or GI symptoms.
Melatonin
Melatonin (N-acetyl-5-methoxytryptamine) is a chronobiological hormone secreted by the pineal gland that is responsible for maintaining circadian rhythms. Melatonin acts as a potent antioxidant, immune modulating agent, antitumor, and antitoxic agent with respect to reducing radiation and chemotherapy induced toxicity (Seely 2011, Vijayalaxmi 2002). A recent meta analysis by Seely et al including 21 RCTs showed that melatonin alongside chemo and/ or radiation therapy improves survival (reduction in one year mortality, RR = 0.60; 95% CI = 0.54-0.67) and decreases toxicities of treatment, including asthenia, leucopenia, nausea and vomiting, hypotension, and thrombocytopenia (2011). Preliminary trials have shown an effect of melatonin on cachexia.
In a randomized controlled trial, 24 patients with advanced gastrointestinal cancer were randomized to receive fish oil or melatonin (Persson 2005). For the fish oil group, 13 patients received the equivalent of 4.9 g EPA and 3.2 g DHA/d, while the melatonin group (n=11) were given 18 mg melatonin/d. After four weeks, five of the 13 patients (38%) in the fish oil group and 3 of 11 patients (27%) in the melatonin group showed weight stabilization or gain. Notably, after combining both interventions, 63% of individuals responded, suggesting an additive effect from the combination.
In another study, Lissoni et al found that among 100 patients with untreatable metastatic solid tumours, those receiving supportive care plus melatonin (20 mg/day orally in the evening x2mo) versus supportive care alone showed slower weight loss and decrease in TNF levels: “The per cent of weight loss greater than 10% was significantly higher in patients treated by supportive care alone than in those concomitantly treated by MLT, with no difference in food intake (P < 0.01)” (1996).
Branched Chain Amino Acids
The branched chain amino acids consist of leucine, isoleucine, and valine, and these may act indirectly by modulating serotonin activity in the hypothalamus. In the brain, the synthesis of neurotransmitters such as serotonin, dopamine, and norepinephrine are dependent upon the availability of the aromatic amino acids (tryptophan, phenylalanine, and tyrosine) respectively (Fernstrom 2005). Branched chain amino acids are thought to counteract the effects of anorexia by competing with tryptophan for entry through the blood brain barrier (Inui 2002). In cancer serotonin levels can be elevated as a result of increased plasma tryptophan. Through competition, BCAAs allow less serotonin in the brain, and result in a reduction in the amount of hypothalamic activity contributing to anorexia (Inui 2002). In addition BCAAs possess anabolic effects peripherally in skeletal muscle (Laviano 2005).
In a double blind prospective study, 28 cancer patients with anorexia were given an oral supplement consisting of branched chain amino acids 4.8 g three times daily for seven days (Le Bricon 1996). In the BCAA- treated group, the incidence of anorexia decreased from 100% to 45% by the end of the study. In the placebo group, the incidence of anorexia remained at 84%. The authors concluded that BCAA supplementation can safely be administered for the treatment of cancer-induced anorexia (Le Bricon 1996).
In a study of patients with intraabdominal adenocarcinoma and receiving total parenteral nutrition (TPN), the effects of a solution containing 19% BCAAs was compared to an isocaloric, isonitrogenous formula containing 50% BCAAs (Hunter 1989). In the high BCAA group, the following outcomes were observed in comparison to the low BCAA group: increase in whole body protein synthesis and breakdown (p<0.05); increased synthesis rate of albumin (P< 0.05); and reduction in tyrosine oxidation, suggesting improved protein utilization.
In terms of BCAA supplementation in increasing lean body mass, clinical trials have been inconsistent in elucidating its effects on skeletal muscle synthesis in humans (Choudry 2006). To date, trials have largely been small and variable and there is no clear positive or negative benefit to BCAA supplementation. The evidence regarding BCAA supplementation in severe catabolic patients is more encouraging. Studies regarding this particular patient population have used more objective outcome measures such as decreases in urinary nitrogen excretion to show benefits.
L-Carnitine
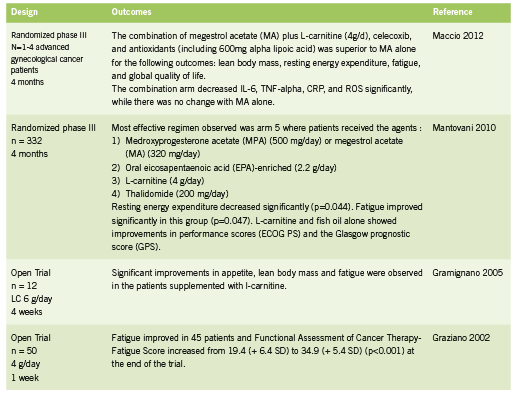
L-carnitine is an amino acid derivative and important cofactor in facilitating mitochondrial fatty acid metabolism within muscle tissue. L-carnitine is responsible for the transportation of fatty acids across the mitochondrial membrane in exchange for acetyl CoA, a byproduct of fatty acid oxidation (Fritz 2011). Maintaining this shuttle system 1. ensures substrate (fatty acids) for the production of ATP through beta oxidation in the mitochondria; and 2. prevents inhibition of glucose metabolism, which could happen through inhibition of pyruvate dehydrogenase by buildup of acetylCoA within the mitochondria (Fritz 2011). Some forms of chemotherapy have been shown to deplete carnitine, in part through impaired renal reabsorption (Hockenberry 2009, Mancinelli 2007). Open label studies of L-carnitine in cancer patients have shown improvements in lean body mass and fatigue (Gramignano 2005, Graziano 2002); RCTs of L-carnitine in combination with other agents in the treatment of cachexia have shown a superior combined effect on measure of cachexia, prognostic scores, and inflammatory cytokines (Maccio 2012, Mantanovi 2010).
Whey Protein Whey protein supplementation contributes to adequate protein intake, while supplying all the essential amino acids in order to maximize muscle protein synthetic activity and optimal immune function. Whey contains high concentrations of the BCAAs as well as beta-lactoglobulin, alpha-lactalbumin, bovine serum albumin, lactoferrin, immunoglobulins, lactoperoxidase enzymes, glycomacropeptides, lactose, and minerals (AltMedRev 2008). Human evidence shows that whey protein is rapidly absorbed and can effectively stimulate muscle protein synthesis (FSR) (Deutz 2011).
Use of a high protein, high leucine whey formula was investigated in 25 cancer patients prior or after chemotherapy treatment (Deutz 2011). The whey group (n = 13) received a formula containing 40g of protein based on casein, whey, and free leucine, while the control group (n =12) was given 24g of casein alone. Results showed that the fractional rate of muscle protein synthesis (FSR) significantly increased from 0.073 (SD: 0.023) to 0.097 (SD: 0.033) (p = 0.0269) in the whey group but not in the control group, showing the superior effects of a whey and leucine containing protein supplement.
A small trial examining the effect of whey on cancer progression was conducted among seven patients with metastatic cancer (five breast, one liver, one pancreas) (Kennedy 1995). Whey protein was supplemented at 30g daily for six months. In six patients, baseline blood lymphocyte GSH (glutathione) levels were substantially elevated, according to authors, “reflecting high tumour GSH levels” (Kennedy 1995). After treatment with whey, two patients exhibited “signs of tumour regression, normalization of haemoglobin and peripheral lymphocyte counts and a sustained drop of lymphocyte GSH levels towards normal” (Kennedy 1995). Two patients showed tumour stabilization and increased haemoglobin levels. Three patients had disease progression. The authors conlcuded that “whey protein concentrate might deplete tumour cells of GSH and [thereby] render them more vulnerable to chemotherapy” (Kennedy 1995). In addition to effects on tumor cell levels of GSH, whey protein has been shown to possess anticancer activity in animals by increasing GSH concentration in healthy tissues, thereby stimulating immune function through the GSH pathway (Bounous 2000). Glutathione is depleted in conditions of immune deficiency, for example HIV and cachexia; under these circumstances, whey protein acts as a cysteine donor to replenish GSH, since cysteine is the crucial limiting amino acid for intracellular GSH synthesis (Bounous 2000).
Conclusion
Naturopathic interventions used for treatment in cancer cachexia are based on good human level evidence and may offer important advantages to cancer patients, particularly in combination. These include high EPA fish oil, melatonin, branched chain amino acids, L-carnitine, and whey protein supplementation. These agents have a good safety profile in that little or no interactions with chemotherapy have been observed in human trials. While evidence suggests benefit from the application of each of these nutrients in isolation, a much stronger impact can reasonably be anticipated from their combined application, thus targeting multiple pathways and factors underlying the pathophysiology of cancer cachexia.
Coauthor Reshi Mehta would like to thank Dr Kieran Cooley, Dr Douglas Andrews, and Dr Philip Rouchotas for their guidance in the compilation of this manuscript.
References:
Altern Med Rev. Whey protein. Monograph. 2008 Dec;13(4):341-7.
Asher V, Lee J, Bali A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med Oncol. 2011 Jul 7.[Epub ahead of print]
Bachmann J, Heiligensetzer M, Krakowski-Roosen H, Büchler MW, Friess H, Martignoni ME. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg. 2008 Jul;12(7):1193-201.
Barber MD, Fearon KCH, Tisdale MJ, McMillan DC, Ross JA. Effect of a Fish Oil- Enriched Nutritional Supplement on Metabolic Mediators in Patients With Pancreatic Cancer Cachexia. NUTRTION AND CANCER 2001 40(2):118-124
Bayram I, Erbey F, Celik N, Nelson JL, Tanyeli A. The use of a protein and energy dense eicosapentaenoic acid containing supplement for malignancy-related weight loss in children. Pediatr Blood Cancer. 2009 May;52(5):571-4.
Bounous G. Whey protein concentrate (WPC) and glutathione modulation in cancer treatment. Anticancer Res. 2000 Nov-Dec;20(6C):4785-92.
Bruera E, Strasser F, Palmer JL, Willey J, Calder K, Amyotte G, Baracos V. Effect of Fish Oil on Appetite and Other Symptoms in Patients with Advanced Cancer and Anorexia/Cachexia: A Double-Blind, Placebo-Controlled Study. J Clin Oncol 2003 Jan;21(1):129-134
Burckart K, Beca S, Urban RJ, Sheffield-Moore M. Pathogenesis of muscle wasting in cancer cachexia: targeted anabolic and anticatabolic therapies. Curr Opin Clin Nutr Metab Care. 2010 Jul;13(4):410-6.
Choudry HA, Pan M, Karinch AM, Souba WW. Branched-chain amino acidenriched nutritional support in surgical and cancer patients. J Nutr. 2006 jan;136(1 Suppl):314S-8S
Churm D, Andrew IM, Holden K, Hildreth AJ, Hawkins C. A questionnaire study of the approach to the anorexia-cachexia syndrome in patients with cancer by staff in a district general hospital. Support Care Cancer. 2009 May;17(5):503-7.
Colomer R, Moreno-Nogueira JM, Garcia-Luna PP, Garcia-Peris P, Garcia-de- Lorenzo A, Zarazaga A, Quecedo L, del Llano J, Usán L, Casimiro C. N-3 fatty acids, cancer and cachexia: a systematic review of the literature. Br J Nutr. 2007 May;97(5):823-31
Deutz NE, Safar A, Schutzler S, Memelink R, Ferrando A, Spencer H, van Helvoort A, Wolfe RR. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin Nutr. 2011 Dec;30(6): 759-68
Donohoe C, Ryan AM, Reynolds JV. Cancer Cachexia: Mechanisms and Clinical Implications. Gastroenterol Res Pract. 2011 Jun;2011:1-13
Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar- Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr. 2008 Dec;27(6):793-9.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011 May;12(5):489-95.
Fearon KC, Barber MD, Moses AG, Ahmedzai SH, Taylor GS, Tisdale MJ, Murray GD. Double-blind, placebo-controlled, randomized study of eicosapentaenoic acid diester in patients with cancer cachexia. J Clin Oncol. 2006 Jul 20;24(21):3401-7.
Fernstrom JD. Branched-chain amino acids and brain function. J Nutr. 2005 Jun;135(6 Suppl):1539S-46S
Fox KM, Brooks JM, Gandra SR, Markus R, Chiou CF. Estimation of Cachexia among Cancer Patients Based on Four Definitions. J Oncol. 2009;2009:693458.
Fritz, H. L-carinitine: cardiovascular applications. IHP February/March 2011: 40-44.
Gramignano G, Lusso MR, Madeddu C, Massa E, Serpe R, Deiana L, Lamonica G, Dessi M, Spiga C, Astara G, Macciò A, Mantovani G. Efficacy of l-carnitine administration on fatigue, nutritional status, oxidative stress, and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutrition. 2006 Feb;22(2):136-45
Graziano F, Bisonni R, Catalano V, Silva R, Rovidati S, Mencarini E, Ferraro B, Canestrari F, Baldelli AM, De Gaetano A, Giordani P, Testa E, Lai V. Potential role of levocarnitine supplementation for the treatment of chemotherapy-induced fatigue in non-anaemic cancer patients. Br J Cancer. 2002 Jun;86(12):1854-7
Gullett N, Rossi P, Kucuk O, Johnstone PA. Cancer-induced cachexia: a guide for the oncologist. J Soc Integr Oncol. 2009 Fall;7(4):155-69.
Hockenberry MJ, Hooke MC, Gregurich M, McCarthy K. Carnitine plasma levels and fatigue in children/adolescents receiving cisplatin, ifosfamide, or doxorubicin. J Pediatr Hematol Oncol. 2009 Sep;31(9):664-9.
Hunter DC, Weintraub M, Blackburn GL, Bistrian BR. Branched chain amino acids as the protein component of parenteral nutrition in cancer cachexia. Br J Surg. 1989 Feb;76(2):149-53.
Inui A. Cancer Anorexia-Cachexia Syndrome: Current Issues in Research and Management. CA Cancer J Clin 2002;52:72-91
Kennedy RS, Konok GP, Bounous G, Baruchel S, Lee TD. The use of a whey protein concentrate in the treatment of patients with metastatic carcinoma: a phase I-II clinical study. Anticancer Res. 1995 Nov-Dec;15(6B):2643-9.
Laviano A, Muscaritoli M, Cascino A, Preziosa I, Inui A, Mantovani G, Rossi-Fanelli F. Branched-chain amino acids: the best compromise to achieve anabolism? Curr Opin Clin Nutr Metab Care. 2005 Jul;8(4):408-14.
Le Bricon T. Effects of administration of oral branched-chain amino acids on anorexia and caloric intake in cancer patients. Clin Nutr. 1996 Dec;15(6):337
Lis CG, Grutsch JF, Vashi PG, Lammersfeld CA. Is serum albumin an independent predictor of survival in patients with breast cancer? JPEN J Parenter Enteral Nutr. 2003 Jan-Feb;27(1):10-5.
Lissoni P, Paolorossi F, Tancini G, Barni S, Ardizzoia A, Brivio F, Zubelewicz B, Chatikhine V. Is there a role for melatonin in the treatment of neoplastic cachexia? Eur J Cancer. 1996 Jul;32A(8):1340-3.
Macciò A, Madeddu C, Gramignano G, Mulas C, Floris C, Sanna E, Cau MC, Panzone F, Mantovani G. A randomized phase III clinical trial of a combined treatment for cachexia in patients with gynecological cancers: evaluating the impact on metabolic and inflammatory profiles and quality of life. Gynecol Oncol. 2012 Mar;124(3):417-25.
MacDonald N, Easson AM, Mazurak VC, Dunn GP, Baracos VE. Understand and managing cancer cachexia. J Am Coll Surg. 2003 Jul;197(1):143-61
Mancinelli A, D’Iddio S, Bisonni R, Graziano F, Lippe P, Calvani M. Urinary excretion of L-carnitine and its short-chain acetyl-L-carnitine in patients undergoing carboplatin treatment. Cancer Chemother Pharmacol. 2007 Jun;60(1):19-26.
Mantovani G. Randomised phase III clinical trial of 5 different arms of treatment on 332 patients with cancer cachexia. Eur Rev Med Pharmacol Sci. 2010 Apr;14(4):292-301.
Murphy RA, Mourtzakis M, Chu QS, Baracos VE, Reiman T, Mazurak VC. Nutritional intervention with fish oil provides a benefit over standard of care for weight and skeletal muscle mass in patients with nonsmall cell lung cancer receiving chemotherapy. Cancer. 2011 Apr 15;117(8):1775-82. A
Murphy RA, Mourtzakis M, Chu QS, Baracos VE, Reiman T, Mazurak VC. Supplementation with fish oil increases first-line chemotherapy efficacy in patients with advanced nonsmall cell lung cancer. Cancer. 2011 Aug 15;117(16):3774-80. B
Naito T, Tashiro M, Yamamoto K, Ohnishi K, Kagawa Y, Kawakami J. Impact of cachexia on pharmacokinetic disposition of and clinical responses to oxycodone in cancer patients. Eur J Clin Pharmacol. 2012 Mar 23. [Epub ahead of print]
Oshima H, Oshima M. The inflammatory network in the gastrointestinal tumor microenvironment: lessons from mouse models. J Gastroenterol. 2012 Feb;47(2):97-106.
Pepersack T. For an operational definition of cachexia. Letter. Lancet Oncol. 2011 May;12(5):423-4.
Persson C, Glimelius B, Rönnelid J, Nygren P. Impact of fish oil and melatonin on cachexia in patients with advanced gastrointestinal cancer: a randomized pilot study. Nutrition 2005 Feb;21(2):170-8.
Polterauer S, Grimm C, Seebacher V, Rahhal J, Tempfer C, Reinthaller A, Hefler L. The inflammation-based Glasgow Prognostic Score predicts survival in patients with cervical cancer. Int J Gynecol Cancer. 2010 Aug;20(6):1052-7.
Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T, Smith IE, O’Brien ME. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer. 2004 May 17;90(10):1905-11.
Seely D, Wu P, Fritz H, Kennedy DA, Tsui T, Seely AJ, Mills E. Melatonin as Adjuvant Cancer Care With and Without Chemotherapy: A Systematic Review and Metaanalysis of Randomized Trials. Integr Cancer Ther. 2011 Oct 21. [Epub ahead of print]
Spiro A, Baldwin C, Patterson A, Thomas J, Andreyev HJ. The views and practice of oncologists towards nutritional support in patients receiving chemotherapy. Br J Cancer. 2006 Aug 21;95(4):431-4.
Thoresen L, Frykholm G, Lydersen S, Ulveland H, Baracos V, Prado CM, Birdsell L, Falkmer U. Nutritional status, cachexia and survival in patients with advanced colorectal carcinoma. Different assessment criteria for nutritional status provide unequal results. Clin Nutr. 2012 Jun 11.
Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009 Apr;89(2):381-410
Vijayalaxmi, Thomas CR Jr., Reiter RJ, Herman TS. Melatonin: from basic research to cancer treatment clinics. J Clin Oncol. 2002 May;20(10):2575-601
Weed HG, Ferguson ML, Gaff RL, Hustead DS, Nelson JL, Voss AC. Lean body mass gain in patients with head and neck squamous cell cancer treated perioperatively with a protein- and energy-dense nutritional supplement containing eicosapentaenoic acid. Head Neck. 2011 Jul;33(7):1027-33.
Wigmore SJ, Berber MD, Ross JA, Tisdale MJ, Fearon KC. Effect of oral eicosapentaenoic acid on weight loss in patients with pancreatic cancer. Nutr Cancer. 2000 36(2):177-84
Yang R, Cheung MC, Pedroso FE, Byrne MM, Koniaris LG, Zimmers TA. Obesity and weight loss at presentation of lung cancer are associated with opposite effects on survival. J Surg Res. 2011 Sep;170(1):e75-83.
Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7(5):651-8.







