Sarcopenia
Nutritional intervention for a quiet epidemic
Sarcopenia is a complex, age related process that occurs in every aging adult patient and is virtually untreatable by pharmaceutical interventions. This article will explain the lack of available treatment and provide evidence for the use of supplemented protein to prevent and reverse muscle loss in elderly patients.
Sarcopenia research has been poor to date due to inconsistencies in diagnostic criteria and the difficulty in isolating the condition from other atrophic diseases such as cachexia and disuse atrophy. Measuring muscle with imaging has proven to be an inaccurate surrogate for muscle strength and power, the factors that account for the morbidity and mortality associated with sarcopenia (Brass 2011). Given these limitations to the research, and the known complexity of the disease, the development of therapeutic interventions has been challenging.
Early theories on the mechanisms behind age related muscle atrophy focused on increased muscle catabolism as a principle cause, however more recent evidence has shown that muscle breakdown in the elderly appears to occur at similar rates to younger cohorts (Koopman 2011). Evidence has shown that the most marked changes in the aging skeletal muscle lays in the anabolic processes responsible for increasing muscle mass in response to nutrition and exercise. The term anabolic resistance was coined to describe these processes, which include: decreasing insulin sensitivity, decreased protein synthesis in response to dietary essential amino acids (EAA) and decreased protein synthesis response to exercise.
Sarcopenia differs in both mechanism and clinical presentation from cachexia. Unlike cachexia, sarcopenia has a relative preservation of fat mass, which may cause weight loss to be masked in many patients. Evidence has shown that weight stable elderly patients should still be considered for treatment of sarcopenia (Gallagher 2000).
Drug trials have focused on increasing anabolic hormones that decline with age (testosterone and other androgens), and improving blood flow to atrophic muscles. Testosterone supplementation to hypogonadal men has shown some benefit, however given the increased risk of cardiovascular events (Basaria 2010), focus has shifted to Selective Androgen Receptor Modulators (SARMs) (Rolland 2011). Growth hormone, ghrenlin, estrogen and myostatin inhibitors have all been investigated for the treatment of sarcopenia without substantial gains in muscle mass (Rolland 2011). ACE inhibitors show promise for the treatment of muscle loss after studies found that patients treated for hypertension with ACEI were found to have a stabilization of muscle atrophy. This benefit is suspected to be due to improved blood flow to peripheral tissues (Brass 2011).
Nutritional supplementation for elderly patients must improve overall under-nutrition with a particular focus on protein intake.
Improving caloric intake alone has been shown to offer no benefit to muscle mass or function in the elderly (Milne 2009). Outlined below is the current evidence for using protein supplements for the prevention of sarcopenia as well as for treatment of reduced muscle mass. When considering protein supplementation in elderly patients factors to account for include: dose, %EAA, protein source, and timing of protein intake relative to other foods.
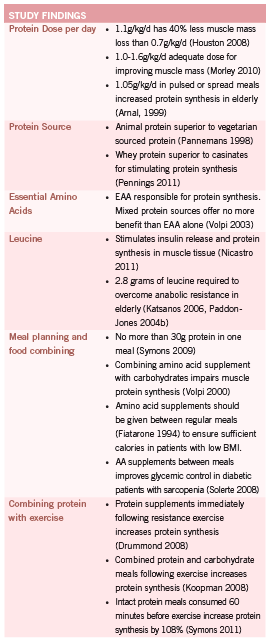
Current recommendations for protein intake in the elderly (0.8g/ kg/d) offer little benefit to sarcopenic patients (Morley 2010). Reviews have shown that many elderly patients do not consume the recommended 0.8g/kg/d (Kerstetter 2003, Rousset 2003) and patients who do consume this dosage are only modestly protected against muscle loss (Genaro 2010, Morley 2010). The Healthy Aging and Body Composition study demonstrated that elderly patients in the highest quartile (protein intake 1.1g/kg/d) lost 40% less appendicular lean mass than patients in the lowest quartile (0.7g/kg/d) (Houston 2008). In a recent review, Morley and colleagues discussed the evidence for increasing the recommended daily dosage of protein for aging adults to 1.0-1.6g/kg/d based on data that shows that 1.0g/kg/d is the minimum dosage required to prevent muscle loss with dosages ranging up to 1.6g/kg/d showing improvements in muscle mass (Morley 2010).
Conflicting studies exist for the timing of protein intake throughout the day as well as the positive or negative impact of combining protein intake with carbohydrates. Arnal et al (Arnal 1999) found that elderly women who consumed the majority of their protein intake at the midday meal had 80% greater protein retention than the control group as calculated by a more positive nitrogen balance. However, in a randomized controlled trial in 2009, Symons et al found that a bolous of 90 grams of animal protein (lean beef) was no more beneficial than a 30 gram bolous through direct examination of protein synthesis through vastus lateralus biopsy (Symons 2009). This more recent study suggests that an upper limit per meal is reached in patients around 30 grams. This study also provides more conclusive evidence given the direct observation of protein synthesis (through uptake of radiolabeled phenylalanine in muscle biopsy) versus calculated protein use based on nitrogen balance. Research has also suggested supplementation of elderly patients with protein between meals to prevent supplementation from replacing calories and nutrients consumed during regular meals (Fiatarone 1994).
The current information on whether to combine protein with carbohydrates requires further research before conclusions can be drawn. Studies on patients assigned to bed-rest have shown that a mixed meal of 16.5g of EAA with 30g of carbohydrates reduced muscle loss, with controls having a more pronounced loss of muscles strength (Paddon-Jones 2004). Volpi and colleagues concluded that combining a protein bolous with carbohydrates impaired protein synthesis in elderly subjects compared to a protein bolus alone (Volpi 2000). Reviews on the subject have concluded that the presence of carbohydrates in modest doses (<30g) do not impair muscle protein synthesis in the elderly however larger doses may negatively influence insulin levels and lower protein synthesis (Kim 2010, Koopman 2011).
Differing protein sources and amino acid ratios produce varying results, illustrating the need for attention and detail when prescribing protein supplements to this population. Protein sources vary in the relative percentages of EAA as well as the speed at which they are digested and increase plasma levels. Whey protein isolates have shown benefit over casein protein mixtures in both the rate of availability of EAA in plasma and in the ability to stimulate protein synthesis in muscle cells (Pennings 2011). However, in a second 2011 study, Dideriksen et al showed no difference in muscle protein synthesis between whey and caseinate ingested after resistance training in the elderly (Dideriksen 2011).
Current evidence shows that animal protein improves lean muscle tissue better than vegetarian sourced protein (Pannemans 1998) and that amino acid supplements containing only essential amino acids (EAA) confer better results than mixed, or non-EAA supplements. A small trial looked at the administration of an EAA only meal to healthy older adults who do not perform exercise on a regular basis (Volpi 2003). Although the size of the trial is a significant limitation, the results clearly indicated that a meal containing 18g of EAA stimulated muscle protein synthesis to the same degree as a 40g meal containing 18g of EAA and 22g of non-essential amino acids.
Of greatest interest, leucine, a branched chain EAA, appears to regulate protein synthesis via multiple processes. Nicastro et al concluded in a 2011 review that leucine stimulates insulin release and is a regulatory molecule for skeletal muscle protein synthesis. Leucine also appears to influence cellular proteolytic processes, which may slow muscle loss (Nicastro 2011). Human controlled trials have shown that leucine enriched meals can improve muscle protein synthesis in the elderly at a dose of 2.8g of leucine in a mixed EAA meal (Katsanos 2006, Paddon-Jones 2004b). There are currently no dose-response trials on leucine supplementation, however the Katsanos trial (Katsanos 2006) was a follow up to a previous study (Katsanos 2005) that found that 1.7g of leucine did not stimulate protein synthesis in elderly subjects compared to younger controls when delivered in a 6.7g mixed protein meal. This possibly indicates a decrease in leucine sensitivity in the elderly with a threshold dose of approximately 2.8g needed to stimulate anabolism. The apparent decrease in leucine sensitivity may be due to age related alterations in first pass metabolism of leucine and other essential amino acids demonstrated by Volpi et al (Volpi 1999).
Much of the research on the adverse outcomes of leucine supplementation has been derived from animal models. The research on possible adverse effects, which include appetite suppression (Nicastro 2011) and imbalances in other branched chain amino acids (Verhoeven 2009) should be considered with caution given the differing needs in individual amino acids from humans to rats and the relatively low dosage of leucine needed in humans to cause a response in skeletal muscle (Nicastro 2011). Trappe et al reported no adverse effects after the administration of 3.6g of leucine in combination with other branched chain amino acids after 60 days (Trappe 2008) to prevent muscle loss during bedrest. In addition, a review by Fernstrom (Fernstrom 2005) found no reported adverse outcomes for supra-physiologic doses of branched chain amino acids in over 20 trials.
The timing of protein intake with respect to resistance exercise has been suggested as a possible treatment option to increase amino acid incorporation into skeletal muscle. In 2011, Pennings et al demonstrated for the first time that de novo protein synthesis is increased in elderly patients who combine protein and exercise versus protein ingestion alone. Subjects completed 30 minutes of stationary bike plus 2 leg resistance exercise before consuming 20g of protein (Pennings 2011b). Although the mechanism behind sarcopenia was thought to include anabolic resistance to exercise, studies have started to show that protein synthesis is increased in the elderly when protein and carbohydrates are ingested following exercise (Koopman 2008), and that the effect of protein ingestion, is delayed, not absent, when compared to younger controls (Drummond 2008).
In a 2011 study by Symons, the discrepancies in the research on the topic of protein and exercise are adequately addressed. In this study, participants were given a large dose of protein (90g) in an intact meal of lean ground beef 60 minutes before exercise. This resulted in a 108% increase in protein synthesis in elderly patients as well as younger controls (Symons 2011). It appears that the timing of protein ingestion should vary, depending on whether patients are consuming intact protein meals (beef, poultry) or quickly digested protein isolates or EAA only supplements. Full meals containing protein require up to 100 minutes to reach peak plasma levels, while protein supplements may elevate plasma AA levels in as little as 15-30 minutes. Timing of exercise and protein prescriptions are thus dependent on the type of protein ingested with intact meals consumed 60 minutes prior to exercise and protein supplements
in a 30-60 minute window post exercise (Symons 2011). Negative studies on protein and exercise have shown no additional benefit to adding exercise to protein intake but either have included subjects who consume >1.0g/kg/d of protein on a regular basis or have used large doses or protein, EAA or leucine over longer periods of time (Koopman 2008, Koopman 2011, Verdijk 2009). This research may suggest that the timing of protein intake with respect to exercise is most valuable as a treatment option in elderly patients who do not consume adequate protein at presentation, but that the benefits of timed doses cease as protein adequacy is reached.
Very few studies have examined protein intake and functional endpoints such as strength. It appears that protein intake is more closely linked to the prevention of appendicular lean mass than muscle strength however more studies are needed to confirm these findings (Scott 2010). One uncontrolled trial examined the use of an EAA+argenine mixture containing 3.95g of leucine and 1.10g of argenine on functional endpoints such as gait speed and maximal leg strength. The study employed only 12 participants but showed significant gains in muscle mass and all functional endpoints measured (Borsheim 2008).
The understanding of sarcopenia will undoubtedly continue to evolve, as already, the use of the term anabolic resistance has begun to shift to account for new research. Recent evidence shows that elderly patients respond to exercise as well as younger controls (Pennings 2011b, Symons 2011) and that the elderly respond similarly to the young after an EAA bolous that exceeds normal dietary intakes (Volpi 1999). It is clear that protein adequacy with a focus on EAA and leucine can prevent muscle wasting and restore muscle mass. Further research into the benefits of protein and sarcopenia are required to determine the effects of carbohydrates and exercise on protein synthesis and functional endpoints such as muscle strength. Sarcopenia is present in the majority of elderly patients and is a condition that can be prevented and possibly treated with careful, specific treatment planning including protein supplementation.
References
Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, Ritz P, Antoine JM, Prugnaud J, Beaufrere B, Mirand PP. Protein pulse feeding improves protein retention in elderly women. Am J Clin Nutr. 1999 Jun;69(6):1202-8.
Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede- Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. N Engl J Med. 2010 Jul 8;363(2):109-22.
Borsheim E, Bui QU, Tissier S, Kobayashi H, Ferrando AA, Wolfe RR. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin Nutr. 2008 Apr;27(2):189-95.
Brass EP, Sietsema KE. Considerations in the development of drugs to treat sarcopenia. J Am Geriatr Soc. 2011 Mar;59(3):530-5.
Dideriksen KJ, Reitelseder S, Petersen SG, Hjort M, Helmark IC, Kjaer M, Holm L. Stimulation of muscle protein synthesis by whey and caseinate ingestion after resistance exercise in elderly individuals. Scand J Med Sci Sports. 2011 Apr 28.
Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield- Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008 May;104(5):1452-61.
Fernstrom JD. Branched-chain amino acids and brain function. J Nutr. 2005 Jun;135(6 Suppl):1539S-46S.
Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994 Jun 23;330(25):1769-75.
Gallagher D, Ruts E, Visser M, Heshka S, Baumgartner RN, Wang J, Pierson RN, Pi-Sunyer FX, Heymsfi eld SB. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab. 2000 Aug;279(2):E366-75.
Genaro Pde S, Martini LA. Effect of protein intake on bone and muscle mass in the elderly. Nutr Rev. 2010 Oct;68(10):616-23.
Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB; Health ABC Study. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008 Jan;87(1):150-5.
Katsanos CS, Kobayashi H, Sheffi eld-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005 Nov;82(5):1065-73.
Katsanos CS, Kobayashi H, Sheffi eld-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006 Aug;291(2):E381-7.
Kerstetter JE, O’Brien KO, Insogna KL. Low protein intake: the impact on calcium and bone homeostasis in humans. J Nutr. 2003 Mar;133(3):855S-861S.
Kim JS, Wilson JM, Lee SR. Dietary implications on mechanisms of sarcopenia: roles of protein, amino acids and antioxidants. J Nutr Biochem. 2010 Jan;21(1):1-13.
Koopman R. Dietary protein and exercise training in ageing. Proc Nutr Soc. 2011 Feb;70(1):104-13.
Koopman R, Verdijk LB, Beelen M, Gorselink M, Kruseman AN, Wagenmakers AJ, Kuipers H, van Loon LJ. Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Br J Nutr. 2008 Mar;99(3):571-80.
Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev. 2009 Apr 15;(2):CD003288.
Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, Deutz NE, Doehner W, Fearon KC, Ferrucci L, Hellerstein MK, Kalantar-Zadeh K, Lochs H, MacDonald N, Mulligan K, Muscaritoli M, Ponikowski P, Posthauer ME, Rossi Fanelli F, Schambelan M, Schols AM, Schuster MW, Anker SD; Society for Sarcopenia, Cachexia, and Wasting Disease. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010 Jul;11(6):391-6.
Nicastro H, Artioli GG, Costa Ados S, Solis MY, da Luz CR, Blachier F, Lancha AH Jr. An overview of the therapeutic effects of leucine supplementation on skeletal muscle under atrophic conditions. Amino Acids. 2011 Feb;40(2):287-300.
Paddon-Jones D, Sheffeild-Moore M, Urban R, Sanford A, Aarsland A, Wolfe R, Ferrando A. Essential Amino Acid and Carbohydrate Supplmentation Ameliorates Muscle Protein Loss in Humans During 28 Days Bedrest. The Journal of Clinical Endorinology and Metabolism. 2004 89(9):4351-4358.
Paddon-Jones D, Sheffi eld-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004b Mar;286(3):E321-8.
Pannemans DL, Wagenmakers AJ, Westerterp KR, Schaafsma G, Halliday D. Effect of protein source and quantity on protein metabolism in elderly women. Am J Clin Nutr. 1998 Dec;68(6):1228-35.
Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr. 2011 May;93(5):997-1005.
Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr. 2011b Feb;93(2):322-31.
Rolland Y, Dupuy C, Abellan van Kan G, Gillette S, Vellas B. Treatment strategies for sarcopenia and frailty. Med Clin North Am. 2011 May;95(3):427-38, ix.
Rousset S, Patureau Mirand P, Brandolini M, Martin JF, Boirie Y. Daily protein intakes and eating patterns in young and elderly French. Br J Nutr. 2003 Dec;90(6):1107-15.
Scott D, Blizzard L, Fell J, Giles G, Jones G. Associations between dietary nutrient intake and muscle mass and strength in community-dwelling older adults: the Tasmanian Older Adult Cohort Study. J Am Geriatr Soc. 2010 Nov;58(11):2129-34.
Solerte SB, Fioravanti M, Locatelli E, Bonacasa R, Zamboni M, Basso C, Mazzoleni A, Mansi V, Geroutis N, Gazzaruso C. Improvement of blood glucose control and insulin sensitivity during a long-term (60 weeks) randomized study with amino acid dietary supplements in elderly subjects with type 2 diabetes mellitus. Am J Cardiol. 2008 Jun 2;101(11A):82E-88E.
Symons TB, Sheffi eld-Moore M, Mamerow MM, Wolfe RR, Paddon-Jones D. The anabolic response to resistance exercise and a protein-rich meal is not diminished by age. J Nutr Health Aging. 2011 May;15(5):376-81.
Symons TB, Sheffi eld-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc. 2009 Sep;109(9):1582-6.
Trappe S, Creer A, Minchev K, Slivka D, Louis E, Luden N, Trappe T. Human soleus single muscle fi ber function with exercise or nutrition countermeasures during 60 days of bed rest. Am J Physiol Regul Integr Comp Physiol. 2008 Mar;294(3):R939-47.
Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ. Infl uence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol. 2011 Mar;300(3):R655-62.
Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, Evans WJ. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am J Physiol Endocrinol Metab. 2002 Mar;282(3):E551-6.
Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH, Wodzig WK, Dendale P, van Loon LJ. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr. 2009 Feb;89(2):608-16.
Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, Dendale P, van Loon LJ. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr. 2009 May;89(5):1468-75.
Vinciguerra M, Musaro A, Rosenthal N. Regulation of muscle atrophy in aging and disease. Adv Exp Med Biol. 2010;694:211-33.
Volpi E, Kobayashi H, Sheffi eld-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003 Aug;78(2):250-8.
Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000 Dec;85(12):4481-90.
Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher fi rst-pass splanchnic extraction. Am J Physiol. 1999 Sep;277(3 Pt 1):E513-20.