Demystifying Bioidentical Hormones
A Review Of BHRT Use In Clinical Practice With Respect To Menopausal Concerns
Abstract
This article provides an overview of the scientific literature regarding the safety and efficacy of bioidentical hormone replacement therapy (BHRT) for the treatment of symptoms associated with menopause, including vasomotor symptoms, urinary tract infections, and vaginal atrophy. This review specifically compares safety of bioidentical estrogens and progesterone to conventional hormone replacement therapy (HRT). Data from large randomized controlled trials such as the Women’s Health Initiative (WHI) and the Heart and Estrogen/progestin Replacement Study (HERS) demonstrate detrimental effects resulting from use of estrogen in combination with synthetic progestins. In contrast, data from the large E3N cohort suggest that bioidentical progesterone may offset this risk. Progesterone differs from synthetic progestins in its biological effects on breast tissue, metabolic parameters, as well as on the cardiovascular system. In addition, different routes of BHRT delivery will be discussed, comparing topical and transdermal application methods with oral administration.
Introduction
Bioidentical hormones are manufactured to be molecularly identical to endogenous human hormones. They are derived primarily from plant sources, such as soy and wild Mexican yam root. The extracted plant compounds then undergo synthetic processing to obtain structures identical to endogenous human hormones (Bosarge 2009). Some examples include estriol, estradiol, estrone, progesterone and testosterone. There are many commercially available, FDA-approved bioidentical hormones in various forms and dosages. The following products are available: in pill form, Estrace or Innofem; vaginal cream for vaginal symptoms such as Estrace; patches such as Alora, Climara, Estraderm; topical gels Estrogel/Elestrin; and ring forms Estring and Fermin. Progesterone forms include in pill form Prometrium, and as vaginal gel Crinone 4%. Combined formulations include: Combipatch (Estradiol and norethindrone acetate), Prefest (pill) and Limara Pro (patch).
By contrast, synthetic hormones differ structurally compared to human hormones. These compounds typically contain a chemically conjugated functional group to prevent the breakdown of the hormone before it reaches the circulation (Bosarge 2009). Examples include conjugated equine estrogen (CEE) or Premarin, and medroxyprogesterone acetate (MPA).
The following diagrams show the molecular structure of biological estrogens and progesterone, compared to the synthetic forms that are used in conventional HRT and are sometimes referred to as “natural hormones”. The bioidentical hormones have a molecular structure identical to the biological hormones.
Rationale for HRT
The use of hormones in the treatment of peri- and postmenopausal symptoms has a long history dating from the 1960s. Hormone replacement therapy (HRT) is often prescribed to treat symptoms associated with menopause, including hot flashes, sleep disturbance and night sweats (Schmidt 2006). However, since the publication of the Women’s Health Initiative Study (WHI) reporting an increased risk of various cardiovascular events and cancers, many women suffering from peri- and post- menopausal symptoms have turned to the use of BHRT. BHRT is perceived as an effective and safer alternative to conventional treatment with synthetic hormones.
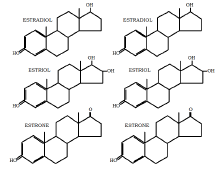
column) compared to synthetics used in conventional
hormone replacement therapy (Right column)
Before menopause, ovarian follicles are the source of greater than 90% of estrogen, particularly estradiol. During pregnancy, estriol is the most active form of estrogen. After menopause, the conversion of androstendione to estrone by non-ovarian tissues is the main source of estrogen. Relief of hot flashes in postmenopausal women can usually be achieved by maintaining serum estradiol levels at 40-50 pg/ml, the lowest level of estradiol that is expected to be seen in a typical menstrual cycle (Schmidt 2006). Co-administration of a progestogen is still the standard method used in order to prevent estrogen-induced endometrial hyperplasia (Emons 2004), but co-administration of a synthetic progestin has its own side effects.
Estrogen receptor subtypes
The conflicting results regarding the safety of estrogen use may be in part due to the different forms of estrogen. Each form exhibits different binding affinity and selectivity to estrogen receptors, which is responsible for mediating a different physiologic effect. Estrogenic effects are mediated through two different estrogen receptor subtypes: estrogen receptor-alpha (ER-a) and estrogen receptor-beta (ER- ). ER-a promotes breast cell proliferation, while ER- inhibits proliferation and prevents breast cancer development via G2 cells cycle arrest (Holtorf 2009).
Estradiol equally activates ER-a and ER- , while estrone selectively activates ER-a at a ratio of 5:1. In contrast, estriol selectively binds ER- at a ratio of 3:1(Holtorf 2009). This suggests that estriol is strongly implicated in breast cancer prevention, while the other forms of estrogens may play a role in breast cancer promotion (Holtorf 2009). Due to minimal (<0.4%) conversion of intravenously administered estradiol or estrone to estriol, given its protective qualities, estriol is physiologically important in postmenopausal women. Therefore it is advisable to co-administer estriol with estradiol (Schmidt 2006).
The synthetic counterpart of endogenous estrogens, CEE (Premarin), also selectively binds ER- . Its components are potent downregulators of the ER- receptor subtype that has anticancer effects. In addition, the concomitant use of synthetic progestins along with CEE synergistically down-regulates ER- receptors (Isaksson 2002), which is another possible mechanism underlying the breast cancer-promoting effect of CEE. CEE also contains at least one particularly potent carcinogenic estrogen, 4-
hydroxy-equilenin, which promotes cancer by inducing DNA damage (Holtrof 2009).
In breast cancer survivors, estrogen therapy is especially controversial. However, breast cancer survivors have a high prevalence of severe menopause symptoms due to the induction of a premature menopause with chemotherapy and/or tamoxifen. It is important to find a therapy that is effective and safe for this patient population (Al-Baghdadi 2009).
Progesterone versus progestins
A review of the studies by Holtorf, Opatrny, and L’Hermite reveals that the use of synthetic progestins is associated with a higher risk of cancer compared to bioidentical progesterone (Holtorf 2009, L’Hermite 2008, Opatrny 2008). In particular, two large randomized controlled trials including the Women’s Health Initiative (WHI) and the Heart and Estrogen/progestin Replacement Study (HERS) have shown that use of CEE accompanied by progestins is associated with increased risk of breast cancer (Chlebowski 2003, Hulley 2002). In the WHI study, risk was increased 24% (HR 1.24, 95% CI 1.01–1.54) compared with placebo after 5.6 years (Chlebowski 2003), while in the HERS study, there was a non-significant 27% increase in risk (HR 1.27, 95% CI 0.84–1.94) compared to non-use after six years (Hulley 2002).
Conversely, important data on the protective effects of bioidentical progesterone comes from the French E3N cohort (Fournier 2005, 2008). In this study, Fournier et al prospectively assessed the risk of breast cancer associated with HRT use in a cohort of over 80,000 postmenopausal women (2005, 2008). Compared to non-use, use of estrogen alone resulted in significantly increased risk of breast cancer, HR 1.29 (95% CI 1.02-1.65) (Fournier 2008). However, in combination regimens, this was modified according to the type of progestagen used. As in the RCTs cited above, breast cancer risk was significantly increased by the use of HRT containing synthetic progestins, however in this study there was no increase with HRT containing micronized progesterone (estrogen-progesterone combination) (Fournier 2008, 2005). For example, when analyzing the risk of breast cancer associated with use of transdermal estrogen, estrogen alone was associated with non-significantly increased breast cancer risk, HR1.28 (0.98–1.69); on the other hand, use of transdermal estrogen in combination with the progestins chlormadinone acetate or medrogestone resulted in significant elevations in beast cancer risk, HR 1.48 (1.05–2.09) and 2.03 (1.39–2.97), respectively. Use of transdermal estrogen in combination with bioidentical progesterone however resulted in minimally changed risk compared to HRT non-use, 1.08 (0.89–1.31). An earlier analysis of this cohort, published in 2005, reported a similar relationship. These 2005 findings are depicted in Figure 2. In conclusion, this study suggests that after a mean 8.1 years follow-up, use of bioidentical progesterone conferred protective effects against breast cancer, compared to HRT utilizing synthetic progestins (Fournier 2008).
In a review by Campagnoli et al, further data is reported to the effect that synthetic androgenic (testosterone-derived) progestins might, when combined to estrogens, increase breast cancer risk through androgen-like effects, for instance by increasing IGF-1 activity (2005). IGF-1 exerts potent mitogenic and anti-apoptotic effects on breast cancer cells, in synergy with estrogens. By comparison, use of continuous bioidentical progesterone (100 mg/day), together with transdermal estradiol, failed to increase IGF-1 concentrations over a six month period (Campagnoli 2005). Table 1 summarizes additional evidence comparing the effects of bioidentical progesterone vs. synthetic progestins, as presented by Holtorf 2009 and Schmidt 2006.
Routes of administration
A few studies have investigated the impact of using transmucosal vaginal estrogen preparations on breast cancer recurrence and mortality. The results of these studies are encouraging, demonstrating that topical estrogen usage was not associated with an increased risk of cancer recurrence; however, the need for randomized, controlled trials was emphasized. The lack of convincing evidence that topical estrogen replacement therapy (ERT) increases recurrence and breast cancer-related mortality may encourage clinicians and patients to consider the less potent
transmucosal vaginal estrogen preparations (Al-Baghdadi 2009).
According to a review by Holtorf, transdermal estradiol, when given with or without oral progesterone, does not have detrimental effects on coagulation or risk for venous thromboembolism (VTE) (2009). In contrast, there is an increased risk for VTE with the use of CEE (both oral and topical with or without synthetic progestin) (Holtorf 2009). According to the ESTHER study, the relative risk of VTE with oral estradiol was significantly higher than transdermal estradiol. Also, estradiol combined with Norpregnane had a significant increased VTE risk compared with estradiol and Pregnane combination. The lowest observed VTE risk was observed in estradiol with micronized progesterone (L’Hermite 2008).
Minimizing side effects: oral versus topical applications
When using HRT, a generally accepted principle is to prescribe the lowest effective dose on an individualized basis. Topical and transdermal formulations permit application without first-pass metabolism, and may reduce the stimulation of liver production of clotting proteins (Schmidt 2006). For instance, markers of blood clotting risk such as activated protein C resistance that are associated with oral estrogen use are not seen with transdermal administration (Oger 2003). Furthermore, while bioidentical hormones can be administered orally, they have poor oral bioavailability. Rapid metabolism by the liver and the hydrophobic nature of sex steroid hormones suggests that topical administration can be used as an alternative (Minkin 2004). According to Schmidt, transdermal administration of estradiol can maintain serum levels within a relatively narrow range for an extended period of time, with relatively low variation in bioavailability between individuals (2006). For progesterone, the large first-pass effect prevents most orally administered progesterone from reaching the blood plasma and generates metabolites with undesirable side effects; and vaginal bioavailability of progesterone is superior to oral (Schmidt 2006).
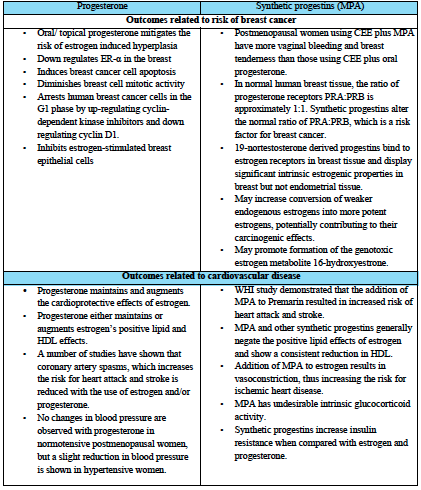
Holtorf 2009 and Schmidt 2006
A 1998 systematic review of 77 studies evaluating estrogen therapy found that while all routes were effective in the treatment of urogenital atrophy, the form with the least systemic absorption was estriol (administered orally or vaginally), followed by vaginal estradiol, as measured by pre- and post- therapy serum estradiol and estrone concentrations (Cardozo 1998). Side effects of local use are typically limited mild burning or pruritus, which generally disappear after several days of treatment (Raz 2001). Intravaginal estriol is well absorbed. Peak plasma levels of unconjugated estriol after insertion of 0.5mg of cream is comparable with those obtained after 8-12mg administered orally.
Estriol replacement is an effective treatment in the treatment of urogenital complaints related to menopause, and may also have a role in the prevention of recurrent UTIs (Cardozo 1998, Raz 2001). Estrogen reduced vaginal pH and stimulates the proliferation of lactobacillus bacteria which compete with Enterobacteriaceae for vaginal colonization, which are the main pathogens of the urinary tract (Raz 2011). The absence of estrogen decreases the volume of vaginal muscles, resulting in slackness of the ligaments holding the uterus, the pelvic floor, and the bladder, resulting in the development of prolapse of the internal genitalia and predisposing to UTI (Raz 2001). The recommended dose is 0.5mg estriol twice weekly after a loading dose, applied for 14 days to vaginal area.
Compounding BHRT
Bioidentical hormones are commercially available in oral or topical preparations. Examples of commercially available estrogens include Estrace® and Climera®. Compounding pharmacists are able to prepare medications from scratch using individual ingredients that are mixed together to formulate a specific strength of medication (Fugh-Berman, 2007). Compounding bioidentical hormones can provide several advantages such as formulating a preparation to meet the specific needs of the patient. Additionally, BHRT’s can be compounded in various forms such as creams, lotions,PLO gels, capsules, suppositories, troches or patches. Precise dosing allows for individualized treatment based on a patient’s symptoms and responses, avoiding unnecessary side effects (Bosarge 2009). For example, BHRTs can be compounded as a cream containing both estriol and estradiol at a minimum effective dose to minimize unwanted side effects. Convenient dosing and reduction of side effects may improve compliance when compared to conventional hormone replacement therapy (Bosarge 2009). Due to the variability of compounding practices between pharmacies, healthcare practitioners and patients should be cautious when choosing where they fill their medication. Compounding pharmacists can take training courses and receive certifications in compounding practice. It is advisable that physicians refer their patient’s to a reputable compounding pharmacy.
Conclusion
The concept of HRT was initiated in the mid-1960s with the goal of alleviating the symptoms of severe menopause and of cushioning against health risks associated with menopause. However, unease over its use surfaced following findings reported by the Women’s Health Initiative (WHI). Bioidentical hormones offer important benefits over conventional hormone replacement therapy. These include a reduced risk of breast cancer with use of progesterone compared with conventional progestin containing regimens, and lack of detrimental cardiovascular effects associated with progestins. Patients often are confused about the optimal approach to hormone replacement therapy. The choice of compounded bio-identical hormones may be appropriate when FDA-approved products do not provide the option of obtaining the desired medication such as transdermal testosterone; or the desired dose, for instance natural progesterone in a dose <100 mg); or when an allergy precludes an FDA-approved product, such as peanut allergy and Prometrium. Informed MDs, NDs, and pharmacists can help their patient make better decisions based on needs, concerns, preferences, and the best available scientific evidence.
Acknowledgements
The authors wish to thank Joyce Wan B.Sc.Phm and Amin Jagani, BPharm, RPh, MBA for their help and contribution to this paper.
References
Al-Baghdadi O., Ewies A.A.A. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric 2009;(12):91-105.4.
Bosarge PM, Freeman S. Bioidentical hormones,
compounding, and evidence-based medicine: what women’s health practitioners need to know. Journal for Nurse Practitioners 2009;5:421-7.
Campagnoli C, Clavel-chapelon F, Kaaks R, Peris C, Berrino F. Progestins and progesterone in hormone replacement therapy and the risk of breast cancer. J Steroid Biochem Mol Biol 2005;96:95-108.
Cardozo L, Bachmann G, McClish D, Fonda D, Birgerson L. Meta-analysis of estrogen therapy in the management of urogenital atrophy in postmenopausal women: second report of the Hormones and Urogenital Therapy Committee. Obstet Gynecol. 1998 Oct;92(4 Pt 2):722-7.
Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, Khandekar J, Petrovitch H, McTiernan A, WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003; 289(24):3243-53.
Emons G, Huschmand-Nia A, Krauss T, Hinney B. Hormone replacement therapy and endometrial cancer. Onkologie 2004;27:207-210. Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies; results from the E3N cohort study. Breast Cancer Res Treat 2008;107:103-11.
Fournier A, Berrino F, RiboliE, Avenel V, Clavel- Chapon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res. Treat. 2008; 107(1):103-111.
Fournier A, Berrino F, Riboli E, Avenel V, Clavel- Chapelon F. Breast cancer risk in relation to different types of hormone replacement therapy in the E3NEPIC cohort. Int J Cancer. 2005 Apr 10;114(3):448-54.
Fugh-Berman A, Bythrow J. Bioidentical hormones for menopausal hormone therapy: variation on a theme. Journal of General Internal Medicine. 2007; 22:1030-1034.
Holtorf K. The bioidentical hormone debate: are bioidentical hormones (estradiol, estriol, and progesterone) safer or more efficacious than commonly used synthetic versions in hormone replacement therapy? Postgrad Med. 2009 Jan;121(1):73-85.
Hulley S, Furberg C, Barrett-Connor E, Cauley J, Grady D, Haskell W, Knopp R, Lowery M, Satterfield S, Schrott H, Vittinghoff E, Hunninghake D, HERS Research Group. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002; 288(1):58-66.
Isaksson E, Wang H, Sahlin L, et al. Expression of estrogen receptors (alpha, beta) and insulin-like growth factor-1 in breast tissue from surgically postmenopausal cynomolgus macaques after long-term treatment with HRT and tamoxifen. Breast. 2002;11(4):295-300.
Leonetti HB, Landes J, Steinberg D, Anasti JN. Transdermal progesterone cream as an alternative progestin in hormone therapy. Altern Ther Health Med 2005;11:36-38.
L’Hermite Marc, Simonici Tommaso, Fuller Sarah, Genazzani Andrea Riccardo. Could transdermal estradiol + progesterone be a safer postmenopausal HRT? A Review. Maturitas 60(2008)185-201.
Minkin MJ. Considerations in the choice of oral vs. transdermal hormone therapy: a review. J Reprod Med 2004;49:311-320.
Mueck AO, Seeger H. Breast cancer: are estrogen metabolites carcinogenic? Climacteric 2007; 10 (Supple.2); 62-5.
Oger E, Alhenc-Gelas M, Lacut K, Blouch MT, Roudaut N, Kerlan V, Collet M, Abgrall JF, Aiach M, Scarabin PY, et al. Differential effects of oral and transdermal estrogen/progesterone regimens on sensitivity to activated protein C among postmenopausal women: a randomized trial. Arterioscler Thromb Vasc Biol 2003;23:1671-1676.
Opatrny L, Dell`Aniello S, Assouline S, Suissa S. Hormone replacement therapy use and variations in the risk of breast cancer. BJOG 2008; 115:169-175.
Raz R., Postmenopausal women with recurrent UTI. International Journal of Antimicrobial Agents 17 (2001) 269-271.
Raz R. Urinary tract infection in postmenopausal women. Korean J Urol. 2011 Dec;52(12):801-8.
Schmidt JW, Wollner D, Curcio J, Riedlinger J, Kim L. S. Hormone replacement therapy in menopausal women: Past problems and future possibilities. Menopause. Gynecological Endocrinology.2006;22(10): 546-577.









