CoQ10 and Heart Failure
A review of evidence
Heart failure is a significant contributor to mortality and morbidity in the U.S. and the developed world. It is typical for heart failure patients to have low levels of coenzyme Q10, which is an integral cofactor for the mitochondrial respiratory chain involved in generating adenosine triphosphate, the major cellular energy source. Coenzyme Q10 is also a potent antioxidant and membranes stabilizer. Since its discovery in 1957, on the basis of a number of studies it has been used therapeutically for heart failure, but this work is largely ignored. Lack of bioavailability is also an issue, but this has been addressed and highly bioavailable products developed. This research is also ignored. It is well known that statin therapy dramatically reduces coenzyme Q10 levels and before the first statin was introduced, inclusion of this coenzyme was considered and rejected. Since coenzyme Q10 is not a prescription drug, most mainstream medical practitioners do not recognize it as a useful supplement or therapeutic agent. Nevertheless, in the context of heart failure, there is evidence that if the blood levels of this cofactor are considerably elevated through supplementation with highly bioavailable products, the impact on the grade of heart failure and the quality of life is highly significant. Beneficial results have been observed when this cofactor is used to treat the side effects of statin treatment and in particular myopathy. The association between dosing levels and outcomes is discussed. There are integrative cardiologists who claim that based on years of clinical experience, they could not effectively practice without this supplement.
Coenzyme Q10 (Q10), also known as ubiquinone, was discovered in 1957 and there followed a considerable amount of research with animals, humans and cell cultures. Many international symposia were held with hard cover proceedings that gather dust on library shelves. Q10 is found in all human cells and is a potent antioxidant, cell membrane stabilizer, and an essential enzyme in the mitochondrial respiratory chain where it is involved in the generation of ATP(Greenberg 1990, Sinatra 2005) Low Q10 has been associated with a number of disorders and its therapeutic use recently reviewed (Villalba 2010). Low levels of Q10 play a role in heart failure, angina and hypertension (Tran 2001). This is important since heart failure contributes significantly to mortality and morbidity in the U.S. and other developed countries. Depending on symptom severity, heart dysfunction, and other factors, in Canada heart failure can be associated with an annual mortality of between 5% and 50% (Arnold 2006). The importance of Q10 for heart function is illustrated by five clinical trials cited by in a recent review with significant improvements found in endpoints of ejection fraction, pulmonary artery pressure, stroke volume, cardiac output, and functional capacity and quality of life associated with supplementation (Lee 2011).
Food is only a minor source of this cofactor. The pathway that leads to the endogenous synthesis of Q10 is also the pathway to cholesterol. Drugs called HMG-CoA reductase inhibitors, better known as statins, inhibit this pathway with the resultant large decrease in both cholesterol and Q10. Concern over the impact of the widespread use of statins in this context is mounting as the pressure intensifies to have everyone on statins, from toddlers to the frail elderly.
Q10 is not viewed with enthusiasm by mainstream medicine, although a systematic review published in 2003 (Rosenfeldt 2003) noted non-significant trends toward increased ejection fraction and reduced mortality associated with Q10 therapy and another study published in 2006 found Q10 enhanced systolic function with HF (Sander 2006). It is not a prescription drug and in the context of heart failure, recent studies can be cited which suggest it is ineffective. As will be discussed below, this conclusion is contrary to a considerable body of older literature and the experience of integrative cardiologists (Sinatra 2005, Sinatra 2009a, Sinatra 2009b). Instead, several anti-hypertension drugs, digoxin and aldosterone receptor antagonists are the standard of practice along with devices that help the heart beat and contract properly. Heart transplants and end-of-life care are discussed in guidelines. Mainstream medicine would no doubt point to the following two recent studies as evidence that Q10 is not an important issue in heart failure.
In 2011 a study was published (Fumagalli 2011) which reported on a small randomized placebo controlled trial involving 67 patients with heart failure (HF) randomized to receive for eight weeks either a placebo plus the usual care or a combination of a Q10 preparation, viewed as of enhanced bioavailability, and creatine plus the usual care. The Q10 dose was 34 mg/day, a rather low dose. Outcomes were exercise tolerance, peak oxygen consumption from an exercise test and what was called a sickness impact profile. Small improvements were found, mostly of no statistical significance or of small and questionable clinical significance. Q10 blood levels were not reported in spite of the novel nature of the Q10 source.
In 2010 a sub-study of the CORONA study examined the impact of rosuvastatin (Crestor) and Q10 levels on heart failure (McMurray 2010). This was an industry-sponsored study with the majority of the investigators having close financial ties to the sponsor. The average age of subjects was about 72. Serum Q10 was measured but the only intervention was with rosuvastatin. All the subjects had significant to severe heart failure. It was observed that patients with lower Q10 levels at baseline were older and had more advanced heart failure (HF). The statin reduced the mean Q10 levels in each of three tertiles of Q10 from 0.49 to 0.35, 0.75 to 0.46 and 1.10 to 0.53 μg/ml respectively. Mortality was significantly higher among patients in the lowest vs. highest Q10 tertile, but the difference was not significant on multivariate analysis and Q10 was not found upon extensive statistical manipulation to be an independent predictor of either worsening or fatal HF, nor did statin treatment result in worse outcomes. The message: concern over statins, Q10 and HF is not justified.
The Q10 levels need to be put in perspective. The range of Q10 serum levels in self-reported normal, healthy individuals is quite large. In one study of healthy individuals, the distribution of blood Q10 levels was found to be <0.4 μg/L, 2%; 0.4-1.6 μg/mL, 81%; and > 1.6 μg/mL 17% (Lu 2007). The age range was infant to 94 years. There was no gender dependence.
A study published in 2008 does not support the view of Q10 as unimportant. This study (Molyneux 2008) examined the relationship between Q10 blood levels and survival among patients with chronic heart failure (CHF). Two hundred thirty six patients, mean age 77, admitted to hospital with HF were followed for a mean of 2.7 years. The mean Q10 blood level was 0.58 μg/mL. They found a significant difference in survival over the period studied (about 4 years) when a cut-point of 0.63 μg/ mL was used (survival of about 65% vs. 45%). Patients below this cut point had a range of Q10 blood levels of 0.11 to 0.63 whereas those above had a range of 0.63 to 1.50 μg/mL.
Important perspective concerning Q10 and HF can be gained by considering the views of Dr. Peter H. Langsjoen, a cardiologist who has been involved on Q10 research since 1985 and has published extensively in this area. He was recently interviewed and the transcript is available on the internet (Langsjoen 2011). He points out that early on, it was believed that if HF patients typically had Q10 levels around 0.5 μg/mL and normal individuals had levels of around 1μg/mL, then when one was trying to treat HF, the use of supplementation to bring the value up to about 1μg/mL was indicated. When this was tried, not much improvement was seen. Further research revealed that there was a significant blood level threshold at about 2.5 μg/ml above which HF patients appeared to have some benefit and severe HF patients were helped by supplementation once the level achieved was greater than 3.5 μg/mL. This was pointed out in a 2008 paper in Biofactors (Langsjoen 2008). Even in an earlier paper (Langsjoen 1999) in the same journal the threshold of > 3.5 μg/mL blood level was discussed and justified. The results of years of research are clearly being ignored.
In the study by Fumagalli et al, Q10 levels were not measured and the dose was very low. It was thus not surprising that small or non-significant results were obtained. The authors were aware of the 1999 paper which recommended and justified therapeutic levels > 3.5 μg/mL for HF patients. But in this study with a novel source of Q10, the levels achieved were not reported and were probably too low. In the study by McMurry et al the range of Q10 serum levels was too low to be of significance in the context of serious HF, especially in multivariate analysis looking for an independent effect and the lowering of Q10 caused by the statin was probably too small to be of significance in this cohort of patients, many with a need for very high levels.
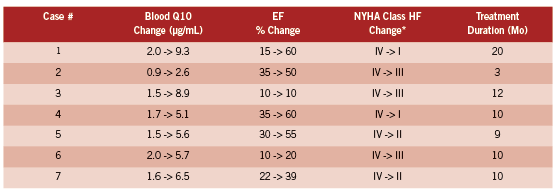
The most interesting study and one which should not have been ignored when the above studies were designed appears to be that of Langsjoen and Langsjoen (Langsjoen 2008). They report on seven consecutive patients who had worsening HF (NYHA Class IV) who were on maximal medical therapy and taking large doses of the ubiquinone form of Q10 which was not, from their point of view concerning the importance of the >3.5 μg/ mL threshold, adequately elevating blood levels in the context of severe HF. Some patients were taking 900 mg/day and still well below the threshold. This could have been written up and presented as a negative study demonstrating that without a doubt Q10 supplementation did not work at all for severe HF. Not so. Patients were switched from an average dose of ubiquinone of 450 mg/day to the ubiquinol form at an average dose of 580 mg/day with a change in average blood Q10 from 1.6 to 6.5 μg/mL. The table below provides the detailed results of ejection fraction (EF) and NYHA class change indicating the decline in the severity of HF after ubiquinol was used rather then ubiquinone. Ubiquinol is the reduced form and the most prevalent form in humans.
The HF class changes listed above are impressive and obviously of huge significance to most of the patients involved. Note the high baseline Q10 levels, which are at the upper extreme of the modern laboratory reference range, and yet the individuals had Class IV HF which was then strongly impacted by changing the supplement to achieve greater bioavailability and thus achieving much higher Q10 levels. Note also the individual variations. This table in fact nicely states the case for treating HF patients to a high target even if their Q10 levels are already high by traditional standards. Four out of seven patients regressed to NYHA I or II. These results suggest the urgent need for a much larger study, but if it has been done it does not appear to have been published.
Even before Langsjoen and Langsjoen carried out their case study, other studies confirmed that while ubiquinone bioavailability was increased when administered in a capsule where the chemical was dispersed in an oil, much higher levels of blood Q10 could be achieved by replacing the oxidized form with ubiquinol (Bhagavan 2007, Hosoe 2007). In fact, Hosoe et al found that supplementation with 300 mg ubiquinol in oil over a period of 28 days raised the Q10 level on average from 0.66 to 7.28 μg/mL. This level was much higher than that achieved with a single dose (2.56 μg/ mL) and thus there is a cumulative effect which must be taken into account in evaluating single dose studies. They found these higher serum levels safe, consistent with a recent safety study (Hidaka 2008). They used Kaneak QH, a Japanese ubiquinol preparation which is available today over the internet from several supplement suppliers. Another comparable preparation is LiQ-NOL CoQ10 which also uses the ubiquinol from Kaneka.
significant limitation of activity due to Symptoms, even to the point of problems walking short distances. Class IV is sufficiently severe as to apply mostly to bed ridden patients.
It has been reported that serious side effects accompany statin use, and that these can be treated and reduced by Q10 supplementation. For example, a small randomized controlled trial reported in 2007 (Caso 2007) was conducted with patients suffering from myopathic symptoms thought to be associated with statin treatment. Supplementation with Q10 (100 mg/day soft-gel) while continuing statin treatment resulted after 30 days in 40% reduction in pain and 38% pain associated interference with daily activities, whereas there were no significant changes in these endpoints in the control group. Similar results were reported in 2005 for patients treated with Q10 after discontinuing statin therapy because of a variety of side effects attributed to this class of drug (Langsjoen 2005).
A more comprehensive alternative approach to heart failure and preventing and treating heart disease in general involves not only Q10 but L-Carnatine and D-Ribose, an approach called Metabolic Cardiology, developed and promoted by the cardiologist Stephen Sinatra on the basis of extensive anecdotal clinical evidence (Sinatra 2005, Sinatra 2009a, Sinatra 2009b). In this context, other micronutrients studied which also appear to provide benefit include omega-3 fatty acids, the B-family of vitamins, and vitamin D (Lee 2011).
A reasonable conclusion appears to be that HF patients are significantly helped by Q10 supplementation, but the dose must be individualized and blood levels measured to test the impact of the intervention. One can not generalize on the oral dose because of the wide variation in both commercial preparations and individual absorption. Thus monitoring and treating to a target serum levels is essential. As pointed out by Langsjoen in the interview cited above, it took some time before it was demonstrated that using doses of 100 mg/day of ubiquinone was not very effective in many cases of HF and early studies found in many cases only small effects. In his interview, Langsjoen is emphatic that there are no side effects or drug interactions to high doses of Q10. He does however point out that Q10 therapy for HF, once heart function improves, reduces the need for some of the “standard treatment” drugs and in particular blood pressure medications. In fact, Q10 had been used to treat hypertension (Wyman 2010).
REFERENCES
Arnold,P., Liu,P., Demers,C., Dorian,P. and Giannetti,N. Canadian Cardiovascular Society consensus conference recommendations on heart failure 2006. Can J Cardiol 2006; 22(1): 23-45.
Bhagavan,H.N. and Chopra,R.K. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion 2007; 7 Suppl: S78-S88.
Caso,G., Kelly,P., McNurlan,M.A. and Lawson,W.E. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol 2007; 99(10): 1409-1412.
Fumagalli,S., Fattirolli,F., Guarducci,L., Cellai,T., Baldasseroni,S., Tarantini,F., Di,B.M., Masotti,G. and Marchionni,N. Coenzyme Q10 terclatrate and creatine in chronic heart failure: a randomized, placebo-controlled, double-blind study. Clin Cardiol 2011; 34(4): 211-217.
Greenberg,S. and Frishman,W.H. Co-enzyme Q10: a new drug for cardiovascular disease. J Clin Pharmacol 1990; 30(7): 596-608.
Hidaka,T., Fujii,K., Funahashi,I., Fukutomi,N. and Hosoe,K. Safety assessment of coenzyme Q10 (CoQ10). Biofactors 2008; 32(1-4): 199-208.
Hosoe,K., Kitano,M., Kishida,H., Kubo,H., Fujii,K. and Kitahara,M. Study on safety and bioavailability of ubiquinol (Kaneka QH) after single and 4-week multiple oral administration to healthy volunteers. Regul Toxicol Pharmacol 2007; 47(1): 19-28.
Langsjoen,P. Congestive heart failure and the clinical uses of coenzyme Q10. Interview by K. R. Hamilton. http://www. prescription2000. com/images/stories/ transcripts/2011-09-08-peter-langsjoen-chf-coenzyme-q10-transcript. pdf 2011.
Langsjoen,P.H. and Langsjoen,A.M. Supplemental ubiquinol in patients with advanced congestive heart failure. Biofactors 2008; 32(1-4): 119-128.
Langsjoen,P.H., Langsjoen,J.O., Langsjoen,A.M. and Lucas,L.A. Treatment of statin adverse effects with supplemental Coenzyme Q10 and statin drug discontinuation. Biofactors 2005; 25(1-4): 147-152.
Langsjoen,P.H. and Langsjoen,A.M. Overview of the use of CoQ10 in cardiovascular disease. Biofactors 1999; 9(2-4): 273.
Lee,J.H., Jarreau,T., Prasad,A., Lavie,C., O’Keefe,J. and Ventura,H. Nutritional assessment in heart failure patients. Congest. Heart Fail. 2011; 17(4): 199-203.
Lu,J. and Frank,E.L. Measurement of coenzyme Q10 in clinical practice. Clin Chim. Acta 2007; 384(1-2): 180-181.
McMurray,J.J., Dunselman,P., Wedel,H., Cleland,J.G., Lindberg,M., Hjalmarson,A., Kjekshus,J., Waagstein,F., Apetrei,E., Barrios,V., Bohm,M., Kamensky,G., Komajda,M., Mareev,V. and Wikstrand,J. Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure: a pre-specified substudy of CORONA (controlled rosuvastatin multinational study in heart failure). J Am Coll Cardiol 2010; 56(15): 1196-1204.
Molyneux,S.L., Florkowski,C.M., George,P.M., Pilbrow,A.P., Frampton,C.M., Lever,M. and Richards,A.M. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. J Am Coll Cardiol 2008; 52(18): 1435-1441.
Rosenfeldt,F., Hilton,D., Pepe,S. and Krum,H. Systematic review of effect of coenzyme Q10 in physical exercise, hypertension and heart failure. Biofactors 2003; 18(1-4): 91-100.
Sander,S., Coleman,C.I., Patel,A.A., Kluger,J. and White,C.M. The impact of coenzyme Q10 on systolic function in patients with chronic heart failure. J Card Fail. 2006; 12(6): 464-472.
Sinatra,S.T., 2005. The Sinatra solution. Basic Health Publications, North Bergen, NJ.
Sinatra,S.T. Metabolic cardiology: an integrative strategy in the treatment of congestive heart failure. Altern. Ther Health Med 2009a; 15(3): 44-52.
Sinatra,S.T. Metabolic cardiology: the missing link in cardiovascular disease. Altern. Ther Health Med 2009b; 15(2): 48-50.
Tran,M.T., Mitchell,T.M., Kennedy,D.T. and Giles,J.T. Role of coenzyme Q10 in chronic heart failure, angina, and hypertension. Pharmacotherapy 2001; 21(7): 797-806.
Villalba,J.M., Parrado,C., Santos-Gonzalez,M. and Alcain,F.J. Therapeutic use of coenzyme Q10 and coenzyme Q10-related compounds and formulations. Expert Opin Investig. Drugs 2010; 19(4): 535-554.
Wyman,M., Leonard,M. and Morledge,T. Coenzyme Q10: a therapy for hypertension and statin-induced myalgia? Cleve. Clin J Med 2010; 77(7): 435-442.