Statin prescription for patients with diabetes
Yeah or Nay?
Introduction
In 2011, the American Diabetes Association (ADA) published their view of the standard of medical care for diabetes (American Diabetes Association 2011). Central to prevention and management of cardiovascular complications is the focus on LDL cholesterol and the recommendation of statin use by a significant fraction of all individuals with diabetes. Is this position consistent with the evidence available?
LDL and Coronary Atherosclerosis. The Absence of an Association Among Individuals with Diabetes
A number of studies have been published recently regarding coronary artery calcium, a marker for atherosclerosis associated coronary plaque, in patients with type II diabetes free of symptomatic cardiovascular disease. All find LDL is not a factor in the prevalence of coronary artery calcium (CAC) (Elkeles 2004, Godsland 2006, Kobayashi 2008, Martin 2009, Mazzone 2007, Wolfe 2002). This conclusion was mostly based on multivariate risk factor analysis that failed to point to LDL as significant. Rather, age, systolic blood pressure or hypertension, gender and race were the important factors for prevalence. These studies, which all involved both genders and a wide range of age and ethnic background, raise a serious issue concerning the total lack of evidence for LDL as a target for therapy associated with coronary atherosclerosis in asymptomatic patients with diabetes. This is important because coronary atherosclerosis is almost always a prerequisite precursor for acute coronary events. These results are consistent with those found in a large number of studies of non-diabetic individuals free of symptomatic heart disease (Ware 2009).
Coronary Artery Calcification. Progression Studies Among Individuals with Diabetes
When coronary calcification is present, it almost always progresses and this is associated with increasing risk of acute cardiac events. LDL also does not appear associated with the risk of progression of CAC among individuals with type II diabetes. Consider the following studies involving subjects free of coronary heart disease (CHD).
• A substudy of the Veterans Affairs Diabetes Trial examined the progression of CAC in 189 individuals with type II diabetes (Saremi 2010). CAC progression was found in > 75% of subjects but was not influenced by standard risk factors and in particular, blood lipids. The albumin to creatinine ratio predicted progression independent of adjustment for age or other traditional risk factors.
• A recent study examined CAC progression in a group of 398 individuals with type II diabetes (Anand 2007). In the final multivariate analysis, odds ratios (OR) for independent factors for progression of CAC were; elevated baseline CAC (OR = 6.38), HbA1c > 7 (OR =1.95), and statin use (OR = 2.27), but not hyperlipidemia or smoking status. It is noteworthy that the researchers found statin therapy failed to inhibit progression of CAC but rather appeared to accelerate it. The increase in risk of progression was 212% for statin-treated patients (38-50%) with LDL < 2.6 mmol/L compared to those untreated. An earlier study from the same group found that 53% of a cohort of individuals with diabetes had subclinical CAC. One-third of these had progression during 2.5 years follow-up (Anand 2006).
• Progression of atherosclerosis over four years measured by CAC was also addressed as part of the PREDICT study (Elkeles 2008). The rate of change was strongly related to the baseline coronary artery calcium score (CACS) and also, independent of CACS, correlated positively with waist to hip ratio, male gender, the use of antihypertensive drugs or statins and the albumin to creatinine ratio. There was no relationship with traditional lipid risk factors.
• Compared to the above, somewhat different results have been reported (Budoff 2005). The study excluded individuals with cardiac symptoms or known CAD including revascularization, stroke or peripheral vascular disease. Two CT calcium scans were done with a mean of 27 months between. Hypercholesterolemia, defined as using cholesterol-lowering drugs or having total cholesterol (TC) ≥ 240 mg/dL, was not found to be a significant risk factor for progression. For the statin non-users, progression was at a median annual rate of 20% (4% to 44%) whereas for statin treated patients it was 10% (4% to 25%) No statistical analysis was included, but the reported ranges are very large.
• It is well known that depression is associated with diabetes and depressive symptoms are a risk factor for CHD (Campayo 2011). A recent report documented this in midlife women in the SWAN Heart Study, where depressive symptoms were independently associated with progression of CAC (Janssen 2011). In a multivariate analysis, the only other predictors of significance for progression were systolic blood pressure and a low level of education but not cholesterol levels.
• Insulin resistance has been independently associated with the progression of CAC. A CAC progression study based on the Kaiser Permanente of Northern California database addressed this issue (Lee 2009). In the univariate analysis TC, LDL, HDL and triglycerides (TGs) were not even close to being significantly associated with progression over two years of follow-up. In multivariate analysis, progression was associated with age, female gender, African American decent, diabetes, fasting insulin, dyslipidemia (presumably high TGs plus low HDL), hypertension, diastolic BP and pulse pressure but not with lipid lowering medication.
In some of these studies, 30-50% of the subjects were on statin therapy at baseline. It is thus curious that statin use also turns up in multivariate analysis in some of these studies to be a positive risk factor. It has been suggested that the positive associations may have been caused by these studies enrolling a high percentage of individuals who had been given statins because they were perceived to be at the greatest risk (Elkeles 2010).
Recently published observational studies, including one that looked at event-free survival, find no association between LDL and cardiovascular/coronary heart disease (CVD/CHD) events among individuals with diabetes (Anand 2006, van Dieren 2011). A recent review even suggested that type 2 diabetes should not be considered a true risk equivalent for CHD (Riche 2007). The consistent finding of no association between TC or LDL cholesterol and the extent or progression of subclinical coronary atherosclerosis is consistent with studies in cohorts of individuals free of diabetes (Ware 2009). Given this, why are LDL targets and lipid lowering with statins central to primary prevention of adverse cardiac events in patients with diabetes? Intervention trials are thus of interest.
Primary and Secondary Prevention of Diabetic Complications with Statins
If one examines the 2011 ADA standard of care (American Diabetes Association 2011), three primary prevention lipid lowering studies are cited where it is possible to stratify both by diabetes and the presence or absence of CHD and CVD, thus obtaining information on the role of statins in risk reduction in the context of primary prevention of acute CHD events in type 2 diabetes. Their Table 11 indicates endpoint restriction to fatal CHD and non-fatal heart attack, but it is extrapolated to 10-year risk. It is thus of interest to look at the individual primary prevention trials. The results were as follows for absolute percent risk reduction (ARR) the numbers needed to treat to prevent one event (NNT), the relative risk reduction (RRR) and the untreated event rate (UTER) for the endpoint of fatal and non-fatal heart attack over 4-5 years (calculated from reported events over the study period). See Table 1.
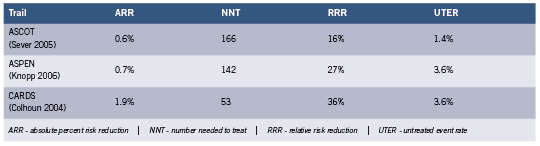
The HPS (MRC/BHF) trial (Collins 2003) is omitted because for the endpoint in question, the study included in the diabetic group individuals with arterial disease, including 33% with previous heart attack or other CHD and 18% with other occlusive artery disease. HPS only stratifies individuals with diabetes by prior CHD when the endpoint includes coronary events, all strokes, and coronary and non-coronary revascularization, which inflates the benefit.
The very small absolute risk reduction (average 1.1%) with large NNT seen in the above table was also found in studies of cohorts where diabetes is present in only a small fraction or absent. When meta-analyses were rigorously restricted to primary prevention statin trials (Wright 2010), absolute risk reduction for major CHD events was 1.0% (11 studies) and the NNT 100. There was no significant impact on mortality. The similarity to cohorts of individuals with diabetes is noteworthy.
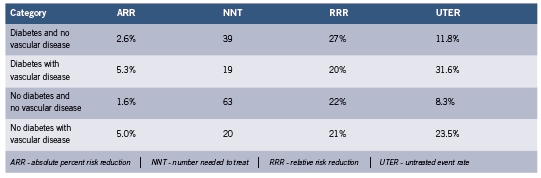
The largest meta-analysis of statin trials where both diabetes and primary vs. secondary prevention were stratified is the Cholesterol Treatment Trialists’ Collaborators (CCT) study. The results are summarized below for the composite endpoint of MI, coronary deaths, stroke, or coronary revascularization over 4-5 years, calculated from reported event numbers (Cholesterol Treatment Trialists’ (CTT) Collaborators 2008). See Table 2. Most participants in these trials were at elevated risk of adverse CVD events.
These results illustrate the greater impact of statins in secondary prevention. The result for individuals with diabetes with no vascular disease is somewhat higher than in CARDS, ASPEN and ASCOT discussed above, presumably because of the expanded endpoint which increases event rates. The results for no diabetes and no vascular disease are slightly higher than those reported above (Wright 2010), again for the same reason.
The CCT results tabulated above which include the NNT should be viewed with some caution since the combined studies average over somewhat different populations. The NNT as it is being used to illustrate clinical utility can only be applied for comparisons when the baseline absolute risks are similar. Also, the NNT decreases dramatically with the duration of studies (Breau 2009, Citrome 2011, McAlister 2008). In the CCT results, duration probably does not confuse the issue.
There is growing suspicion that a significant and perhaps major part of the action of statin drugs involves non lipid lowering (pleiotropic) effects, of which there is a long and impressive list containing actions that can influence acute events but have nothing to do with lower circulating cholesterol (Liao 2005, Mihos 2010, Sadowitz 2010). This significantly impacts the use of LDL lowering trials to prove that LDL is a causative factor. Some might argue that for secondary prevention it does not matter what the statin mechanism is as long as there is a modest (5%) absolute benefit.
Then there are the statin adverse effects. For liver dysfunction, cataracts and myopathy, the number needed to treat to harm one patient with statin therapy has been estimated for men at 142, 52 and 91 and for women 136, 33 and 259, respectively (Hippisley-Cox 2010). It is doubtful that cataracts disappear if statin therapy is terminated. It is also strongly suspected that adverse events are significantly under-reported, downplayed or prevalence data suppressed. The point at which the ARR is large enough and the adverse side effects small enough to justify treatment is of course debatable, and the attitudes of both patient and physician highly variable. Conservative clinicians might conclude that the treatment is not justified in general for very small ARRs, especially in the absence of symptomatic heart disease (Wright 2010)
Conclusions
Thus the obvious question. Does targeting LDL among individuals with diabetes free of cardiovascular disease make any sense when it rarely appears as significant if one examines correlations with the prevalence or progression of silent coronary atherosclerosis or coronary event free survival, or when one takes into account the very small absolute benefits seen in true primary prevention statin intervention trials? Furthermore, optimum LDL targets associated with guideline recommendations for diabetics have been described as not evidence based (Rutter 2011).
LDL is universally regarded as the “bad” cholesterol. If it is so bad, why is it consistently absent as a factor in studies of the risk of prevalence and progression of silent atherosclerosis, the prerequisite precursor of acute events? Statins are ineffective in this context for individuals free from diabetes (Henein 2010, Ware 2009), and individuals with diabetes as discussed above. Why does low or very low LDL characterize half of hospital admissions for heart attack (Al-Mallah 2009)? Why do the non-statin drugs that lower LDL not reduce the event risk, even in secondary prevention (Krumholz 2010)? The simple answer is that the association between heart disease and cholesterol is very much weaker than the conventional wisdom has led us to believe. A new paradigm is needed (de Lorgeril 2009, Ravnskov 2009, Rosch 2008).
The focus on relative rather than absolute risk reduction has resulted in widespread inflated perceptions of benefit, confused the risk/benefit analysis and produced a false sense of security among the millions of statin users. Note the disconnect between ARR and RRR in the above tables. A 20-30% relative risk reduction is impressive and strongly influences therapeutic decisions, but it can be associated with a 1%-2% or even smaller absolute event risk reduction. Patients need to be advised on the basis of absolute risk reductions. A strong case for this was made by Professor Peter Sawicki MD, PhD, from Köln in an invited lecture during a symposium on the cholesterol hypothesis (co-chaired by the author of this review) at the annual meeting of the European Association for the Study of Diabetes recently held in Lisbon, Portugal.
References
Al-Mallah,M.H., Hatahet,H., Cavalcante,J.L. and Khanal,S. Low admission LDLcholesterol is associated with increased 3-year all-cause mortality in patients with non ST segment elevation myocardial infarction. Cardiol. J 2009; 16(3): 227-233.
American Diabetes Association Standards of medical care in diabetes–2011. Diabetes Care 2011; 34 Suppl 1: S11-S61.
Anand,D.V., Lim,E., Darko,D., Bassett,P., Hopkins,D., Lipkin,D., Corder,R. and Lahiri,A. Determinants of progression of coronary artery calcification in type 2 diabetes role of glycemic control and inflammatory/vascular calcification markers. J Am Coll Cardiol. 2007; 50(23): 2218-2225.
Anand,D.V., Lim,E., Hopkins,D., Corder,R., Shaw,L.J., Sharp,P., Lipkin,D. and Lahiri,A. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J 2006; 27(6): 713-721.
Breau,R.H., Fergusson,D. and Dahm,P. Evidence-based urology in practice: number needed to treat. BJU Int 2009; 104(1): 6-8.
Budoff,M.J., Yu,D., Nasir,K., Mehrotra,R., Chen,L., Takasu,J., Agrawal,N., Liu,S.T. and Blumenthal,R.S. Diabetes and progression of coronary calcium under the influence of statin therapy. American Heart Journal 2005; 149(4): 695-700.
Campayo,A., Gomez-Biel,C. and Lobo,A. Diabetes and Depression. Current Psychiatry Reports 2011; 13(1): 26-30.
Cholesterol Treatment Trialists’ (CTT) Collaborators Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. The Lancet 2008; 371(9607): 117-125.
Citrome,L. Number needed to treat: what it is and what it isn’t, and why every clinician should know how to calculate it. J Clin Psychiatry 2011; 72(3): 412-413.
Colhoun,H.M., Betteridge,D.J., Durrington,P.N., Hitman,G.A., Neil,H.A., Livingstone,S.J., Thomason,M.J., Mackness,M.I., Charlton-Menys,V. and Fuller,J.H. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebocontrolled trial. Lancet 2004; 364(9435): 685-696.
Collins,R., Armitage,J., Parish,S., Sleigh,P. and Peto,R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003; 361(9374): 2005-2016.
de Lorgeril,M. Disappointing recent cholesterol-lowering drug trials: is it not time for a full reappraisal of the cholesterol theory? http://michel. delorgeril. info/dwnl/wrnd/ DisappointingCholDrugTrials_wrnd2009. pdf). World Rev Nutr Diet 2009; 100: 80-89.
Elkeles,R.S. Coronary artery calcium and cardiovascular risk in diabetes. Atherosclerosis 2010; 210(2): 331-336. Elkeles,R.S., Feher,M.D., Flather,M.D., Godsland,I.F., Nugara,F., Richmond,W., Rubens,M.B. and Wang,D. The association of coronary calcium score and conventional cardiovascular risk factors in Type 2 diabetic subjects asymptomatic for coronary heart disease (The PREDICT Study). Diabet. Med 2004; 21(10): 1129-1134.
Elkeles,R.S., Godsland,I.F., Rubens,M.B., Feher,M.D., Nugara,F. and Flather,M.D. The progress of coronary heart disease in Type 2 diabetes as measured by coronary calcium score from electron beam computed tomography (EBCT): the PREDICT study. Atherosclerosis 2008; 197(2): 777-783.
Godsland,I.F., Elkeles,R.S., Feher,M.D., Nugara,F., Rubens,M.B., Richmond,W., Khan,M., Donovan,J., Anyaoku,V. and Flather,M.D. Coronary calcification, homocysteine, C-reactive protein and the metabolic syndrome in Type 2 diabetes: the Prospective Evaluation of Diabetic Ischaemic Heart Disease by Coronary Tomography (PREDICT) Study. Diabet. Med 2006; 23(11): 1192-1200.
Henein,M.Y. and Owen,A. Statins moderate coronary stenoses but not coronary calcification: Results from meta-analyses. Int J Cardiol. 2010; Published ahead of print, September 4, 2010.
Hippisley-Cox,J. and Coupland,C. Individualising the risks of statins in men and women in England and Wales: population-based cohort study. Heart 2010; 96(12): 939-947.
Janssen,I., Powell,L.H., Matthews,K.A., Cursio,J.F., Hollenberg,S.M., Sutton-Tyrrell,K., Bromberger,J.T. and Everson-Rose,S.A. Depressive symptoms are related to progression of coronary calcium in midlife women: The Study of Women’s Health Across the Nation (SWAN) Heart Study. Am Heart J 2011; 161(6): 1186-1191.
Knopp,R.H., d’Emden,M., Smilde,J.G. and Pocock,S.J. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care 2006; 29(7): 1478-1485.
Kobayashi,S., Oka,M., Maesato,K., Ikee,R., Mano,T., Hidekazu,M. and Ohtake,T. Coronary Artery Calcification, ADMA, and Insulin Resistance in CKD Patients. Clinical Journal of the American Society of Nephrology 2008; 3(5): 1289-1295.
Krumholz,H.M. and Hayward,R.A. Shifting views on lipid lowering therapy. BMJ 2010; 341: c3531.
Lee,K.K., Fortmann,S.P., Fair,J.M., Iribarren,C., Rubin,G.D., Varady,A., Go,A.S., Quertermous,T. and Hlatky,M.A. Insulin resistance independently predicts the progression of coronary artery calcification. American Heart Journal 2009; 157(5): 939-945.
Liao,J.K. and Laufs,U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 2005; 45: 89-118.
Martin,S.S., Qasim,A.N., Mehta,N.N., Wolfe,M., Terembula,K., Schwartz,S., Iqbal,N., Schutta,M., Bagheri,R. and Reilly,M.P. Apolipoprotein B but not LDL cholesterol is associated with coronary artery calcification in type 2 diabetic whites. Diabetes 2009; 58(8): 1887-1892.
Mazzone,T., Meyer,P.M., Kondos,G.T., Davidson,M.H., Feinstein,S.B., D’Agostino,R.B., Sr., Perez,A. and Haffner,S.M. Relationship of traditional and nontraditional cardiovascular risk factors to coronary artery calcium in type 2 diabetes. Diabetes 2007; 56(3): 849-855.
McAlister,F.A. The “number needed to treat” turns 20–and continues to be used and misused. CMAJ 2008; 179(6): 549-553.
Mihos,C.G., Salas,M.J. and Santana,O. The pleiotropic effects of the hydroxy-methylglutaryl- CoA reductase inhibitors in cardiovascular disease: a comprehensive review. Cardiol. Rev 2010; 18(6): 298-304.
Ravnskov,U. and McCully,K.S. Review and Hypothesis: Vulnerable plaque formation from obstruction of Vasa vasorum by homocysteinylated and oxidized lipoprotein aggregates complexed with microbial remnants and LDL autoantibodies. Ann Clin Lab Sci 2009; 39(1): 3-16.
Riche,D.M. and McClendon,K.S. Role of statins for the primary prevention of cardiovascular disease in patients with type 2 diabetes mellitus. Am J Health Syst. Pharm. 2007; 64(15): 1603-1610.
Rosch,P.J. Cholesterol does not cause coronary heart disease in contrast to stress. Scand Cardiovasc J 2008; 42(4): 244-249.
Rutter,M.K. and Nesto,R.W. Blood pressure, lipids and glucose in type 2 diabetes: how low should we go? Re-discovering personalized care. Eur Heart J 2011. Sadowitz,B., Maier,K.G. and Gahtan,V. Basic science review: Statin therapy–Part I: The pleiotropic effects of statins in cardiovascular disease. Vasc Endovascular. Surg 2010; 44(4): 241-251.
Saremi,A., Moritz,T.E., Anderson,R.J., Abraira,C., Duckworth,W.C. and Reaven,P.D. Rates and determinants of coronary and abdominal aortic artery calcium progression in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 2010; 33(12): 2642-2647.
Sever,P.S., Poulter,N.R., Dahlof,B., Wedel,H., Collins,R., Beevers,G., Caulfield,M., Kjeldsen,S.E., Kristinsson,A., McInnes,G.T., Mehlsen,J., Nieminen,M., O’Brien,E. and Ostergren,J. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial–lipid-lowering arm (ASCOT-LLA). Diabetes Care 2005; 28(5): 1151-1157.
van Dieren,S., Nothlings,U., Van der Schouw,Y.T., Spijkerman,A.M., Rutten,G.E., van der,A.D., Sluik,D., Weikert,C., Joost,H.G., Boeing,H. and Beulens,J.W. Non-fasting lipids and risk of cardiovascular disease in patients with diabetes mellitus. Diabetologia 2011; 54(1): 73-77.
Ware,W.R. The mainstream hypothesis that LDL cholesterol drives atherosclerosis may have been falsified by non-invasive imaging of coronary artery plaque burden and progression. Med Hypotheses 2009; 73(4): 596-600.
Wolfe,M.L., Iqbal,N., Gefter,W., Mohler,E.R., III, Rader,D.J. and Reilly,M.P. Coronary artery calcification at electron beam computed tomography is increased in asymptomatic type 2 diabetics independent of traditional risk factors. J Cardiovasc Risk 2002; 9(6): 369-376.
Wright,J. Do statins have a role in primary prevention? An update. Theraeutics Initative (Theraputics Letter) 2010; 77(March-April 2010, http://ti.ubc.ca/letter77).









