Pregnancy Morbidity
Update on Antiphospholipid Syndrome (APS)
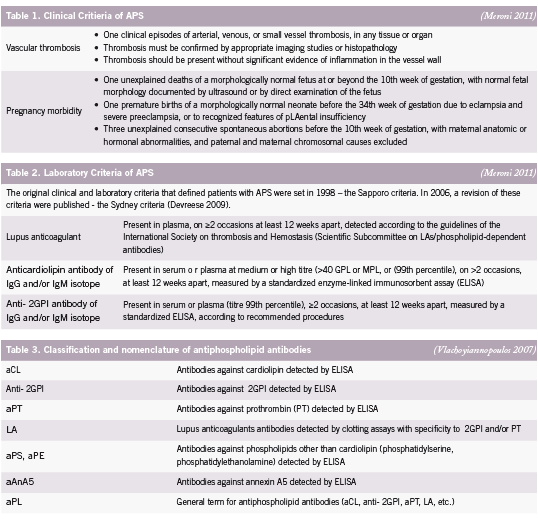
e antiphospholipid syndrome (APS) is an autoimmune disease de ned by two major elements: the occurrence of clinical features, categorised as recurrent vascular thrombosis and/or pregnancy morbidity, and the presence of antiphospholipid antibodies (aPL) in plasma (Devreese 2009). Antiphospholipid syndrome is sometimes referred to as Hughes syndrome, after the rheumatologist Dr. Graham R.V. Hughes. Clinical criteria for APS includes pregnancy loss – either as miscarriage, intrauterine fetal death or stillbirth – and/or obstetric complications, such as intrauterine growth restriction (IUGR), uteroplacental insu ciency, pre-eclampsia and pre-term birth (before 30 weeks) (Carp 2008, Yasuda 1995) (See Table 1). Signi cant associations between recurrent miscarriages and pregnancy complications have been reported with each autoantibody, detectable by di erent assays, in the aPL family including lupus anticoagulant (LA), anti-cardiolipin (aCL) and anti-beta2 glycoprotein I (β2GPI) (Tincani 2003) (See Table 2). Assays of the various aPL are not only diagnostic of APS, but are believed to have a pathogenic role, mediating several clinical manifestations of the syndrome (Meroni 2010).
Antibody Measurements in APS
Antiphospholipid syndrome was rst identi ed more than 20 years ago when the lupus anticoagulant (LA), known to prolong prothrombin time (PT), was found in patients with a history of thrombosis and recurrent second trimester pregnancy loss (Tincani 2003). It is currently considered more appropriate to assess a combination of antibodies rather than one antibody for adequate laboratory detection of APS as no antibody is pathognomic for pregnancy loss or reproductive complications (Carp 2008) (See Table 3).
LA recognition in particular has several disadvantages. Handling and preparation of plasma samples is complicated and labourintensive (Devreese 2009).
e diagnostic value of the aCL antibody test is currently under debate, partly because of methodological problems (Devreese 2009); however, aCL have been shown to help predict cases of Systemic lupus erythematosus (SLE) shifting to secondary APS (Harel 2006).
Antibodies against β2GPI seem to be particularly important, especially when aCL and LA are negative and APS is strongly suspected (Bas de Laat 2004, de Groot 2005). In 3% to 10% of APS patients, anti-β2GPI antibodies might be the only positive test (Ebeling 2003, Lee 2003, Nash 2004). e anti-β2GPI antibody ELISAs (Enzyme-linked immunosorbent assay) detect all antibodies reactive with the protein, making it less speci c, and probably not suitable as a general diagnostic test (de Laat 2008).
Combining the pro le for all three of the tests may enable correct interpretation of results and clinical management (Pengo 2007). A full positive pro le (ie. presence of LA, aCL and β2GPI antibodies) is associated with thrombosis and obstetric complications and appears to re ect the presence of large amounts of antibodies speci cally against β2GPI. LA shows the strongest correlation with thrombosis and pregnancy morbidity (Galli 2003). Patients positive for aCL and β2GPI antibodies, with no history of thromboembolic complications, presented only with obstetric complications (Pengo 2005, Ru atti 2006).
Pathogenic Mechanisms
The thrombophillic e ect of aPL in intraplacental thrombosis, impairing maternal-fetal blood exchange, was originally thought to be the main pathogenic mechanism of fetal loss (Meroni 2010). Histopathological ndings failed to nd thrombosis as the cause of all reproductive failure in samples of miscarried fetuses and placentas from women with APS however (Park 2006). Though thrombosis is indeed a part of the pathophysiology, it is well recognized that different mechanisms are responsible for the vascular and the obstetrical manifestations of APS (Meroni 2010).
Thrombosis
In vivo models of thrombosis induced in mice and hamsters have confirmed that aPL are able to increase thrombus formation in venous and arterial trees (Fischetti 2005, Jankowski 2003,Ramesh 2011). Infusion with and without β2GPI revealed altered expression of endothelial adhesion molecules, and an upregulated expression of nitric oxide and tissue factor (TF) have been reported in arterial endothelia, supporting a key role for aPL in causing vascular abnormalities (Ramesh 2011, Romay- Penabad 2011, Vega-Ostertag 2007). It is the β2GPI, of all the aPL subpopulations, that appears to be most responsible for the thrombotic manifestations of APS (Meroni 2010).
Impaired Trophoblast Invasiveness
Trophoblast invasion is a dynamic process that is tightly controlled by a complex series of interactions between trophoblast and decidual tissues; defective placentation may occur as a result of default in any of them (Meroni 2010, Pierangeli 2008). The differentiation of the trophoblast into an invasive phenotype is related to the expression of cell surface adhesion and signalling molecules (Aplin 2000, Castellucci 2000). Placental invasion might then be affected by abnormal integrin and cadherin expression caused by aPL activity (Di Simone 2002). The failure to express the correct adhesion molecule phenotype by the trophoblast is the disruption that is thought to prevent proper decidual invasion (Meroni 2004). These mechanisms have been suggested to play a role in early fetal loss, while thrombotic events would be responsible for miscarriages late in pregnancy (Meroni 2004).
Uncontrolled Inflammatory Balance
Evidence for the delicate balance between proinflammatory and anti-inflammatory mediators during gestation on the outcome of pregnancy exist (Chaouat 2007). Proinflammatory mediators (such as complement, tumor necrosis factor [TNF], and CC chemokines) have been shown to have a role in animal models of aPL-induced fetal loss (Meroni 2010).
Endogenous Protective Mediator
Annexin V or placental anti-coagulant protein I (PAP-I) is a protein that is expressed on the surface of villous syncitiotrophoblasts. It displays a strong anticoagulant activity in APS by binding to the negatively charged phospholipids, thereby producing a protective shield against aPLs that would otherwise bind to these same phospholipids (Tincani 2003). In APS patients, a decreased quantity of Annexin V has been reported at the placental level (Krikun 1994).
Conventional Treatment of APS
In spite of the general consensus on the management of pregnant aPL-positive women, few well-designed clinical trials have been reported and there is also insufficient data about the positive predictive value (PPV) of treatment (Carp 2008). To date, concomitant administration of heparin and aspirin are used in the treatment of APS. This followed a 1992 controlled trial using 20,000 U/day heparin and 81 mg aspirin that revealed improved pregnancy outcome (Cowchock 1992). The rationale for heparin use was originally founded on the pathogenic role of thrombotic phenomena in aPL-associated pregnancy loss (Hughes 1983), but it was the anticomplement, rather than the anticoagulant, effect of heparin that was later credited in experimental models with mice for the positive pregnancy outcomes (Girardi 2004).
Aspirin has been shown to stimulate Interleukin-3 (IL-3) production (Fishman 1995). IL-3 is a known growth factor for the trophoblast (Wegmann 1989) and IL-3 levels are reduced in women diagnosed with APS during pregnancy (Fishman 1996). In vivo studies in experimental models revealed compelling results such as the elimination of aPL-related obstetrical complications through the addition of exogenous IL-3 (Fishman 1993) that upheld the continued use of aspirin in APS. Some in vitro studies however were unable to provide evidence that IL-3 prevented aPL effect on the trophoblast (Chamley 2001), while others could (Di Simone 2000).
Low corticosteroid doses (<20 mg/day) are occasionally used, particularly in women unresponsive to the standard low-dose aspirin and heparin therapy (Meroni 2011), but there is no sound evidence to support the routine use of corticosteroids in APS (Cowchock 1992, Ruiz-Irastorza 2010). Intravenous immunoglobulin use as a prophylactic treatment of fetal loss in APS patients has been discussed (Tincani 2003). This treatment, added to conventional heparin/aspirin treatment, was unable to improve reproductive prognosis in APS patients (Branch 2000). It is thought that such treatment should be reserved for cases in which conventional therapy has failed or to treat acute problems of pregnancy, especially acute problems of pregnant APS patients (Tincani 2003).
Populations at Greatest Risk for APS
The link between the presence of anti-thyroid antibodies and either isolated or recurrent pregnancy loss has been reproducibly demonstrated (Bussen 1995, Glinoer 1991, Singh 1995, Stagnaro-Green 1990). Presence of anti-thyroid antibodies are significantly correlated with an increased rate of miscarriage in women in their first trimester of pregnancy (Stagnaro-Green 1990) and an increased rate of spontaneous pregnancy loss was reported in women with detectable thyroid autoantibodies compared with controls (Glinoer 1991). Approximately one-third of women diagnosed with APS had anti-thyroid antibodies in a study by De Carolis and colleagues. Women with anti-thyroid antibodies (associated or not with aPL) had significantly lower percentages of pregnancies, and the overall success rate of pregnancy in patients positive for thyroid autoimmunity (alone or association with aPL) was 59% (De Carolis 2004). The presence of anti-thyroid antibodies have an overall PPV of 40% for pregnancy loss (Carp 2008).
That women with systemic lupus erythematosus (SLE) are at greater risk of experiencing pregnancy loss is nothing new. SLE has been known for decades to predispose to spontaneous abortions (Gleicher 1999). It is now known, however, that the risk for pregnancy loss precedes the clinical occurrence and/or diagnosis of SLE as well (Hardy 1999). This bidirectional phenomena is termed reproductive autoimmune failure syndrome (RAFS) and is thought to extend to autoimmune conditions beyond SLE (Gleicher 1999). Many authors suggested that, in addition to other causes, infertile patients might suffer from subclinical autoimmune disease and, in turn, those subclinical autoimmune patients could be at risk of implantation failure or of early post-implantation loss (Geva 1997).
References
Aplin JD, Haigh T, LAey H, Chen CP and Jones CJ. Tissue interactions in the control of trophoblast invasion. J Reprod Fertil Suppl. 2000; 55 57-64.
Bas de Laat H, Derksen RH and de Groot PG. beta2-glycoprotein I, the playmaker of the antiphospholipid syndrome. Clin Immunol. 2004; 112 (2): 161-168.
Branch DW, Peaceman AM, Druzin M, Silver RK, El-Sayed Y, Silver RM, Esplin MS, Spinnato J and Harger J. A multicenter, pLAebo-controlled pilot study of intravenous immune globulin treatment of antiphospholipid syndrome during pregnancy. The Pregnancy Loss Study Group. Am J Obstet Gynecol. 2000; 182 (1 Pt 1): 122-127.
Bussen S and Steck T. Thyroid autoantibodies in euthyroid non-pregnant women with recurrent spontaneous abortions. Hum Reprod. 1995; 10 (11): 2938-2940.
Carp HJ, Meroni PL and Shoenfeld Y. Autoantibodies as predictors of pregnancy complications. Rheumatology. 2008; 47 Suppl 3 iii6-8.
Castellucci M, De Matteis R, Meisser A, Cancello R, Monsurro V, Islami D, Sarzani R, Marzioni D, Cinti S and Bischof P. Leptin modulates extracellular matrix molecules and metalloproteinases: possible implications for trophoblast invasion. Mol Hum Reprod. 2000; 6 (10): 951-958.
Chamley LW, Konarkowska B, Duncalf AM, Mitchell MD and Johnson PM. Is interleukin-3 important in antiphospholipid antibody-mediated pregnancy failure? Fertil Steril. 2001; 76 (4): 700-706.
Chaouat G. The Th1/Th2 paradigm: still important in pregnancy? Semin Immunopathol. 2007; 29 (2): 95-113.
Cowchock FS, Reece EA, Balaban D, Branch DW and Plouffe L. Repeated fetal losses associated with antiphospholipid antibodies: a collaborative randomized trial comparing prednisone with low-dose heparin treatment. Am J Obstet Gynecol. 1992; 166 (5): 1318- 1323.
De Carolis C, Greco E, Guarino MD, Perricone C, Dal Lago A, Giacomelli R, Fontana L and Perricone R. Anti-thyroid antibodies and antiphospholipid syndrome: evidence of reduced fecundity and of poor pregnancy outcome in recurrent spontaneous aborters. Am J Reprod Immunol. 2004; 52 (4): 263-266.
de Groot PG and Derksen RH. Pathophysiology of the antiphospholipid syndrome. J Thromb Haemost. 2005; 3 (8): 1854-1860.
de Laat B, Mertens K and de Groot PG. Mechanisms of disease: antiphospholipid antibodiesfrom clinical association to pathologic mechanism. Nat Clin Pract Rheumatol. 2008; 4 (4): 192-199.
Devreese K and Hoylaerts MF. Laboratory diagnosis of the antiphospholipid syndrome: a plethora of obstacles to overcome. Eur J Haematol. 2009; 83 (1): 1-16.
Di Simone N, Caliandro D, Castellani R, Ferrazzani S and Caruso A. Interleukin-3 and human trophoblast: in vitro explanations for the effect of interleukin in patients with antiphospholipid antibody syndrome. Fertil Steril. 2000; 73 (6): 1194-1200.
Di Simone N, Castellani R, Caliandro D and Caruso A. Antiphospholid antibodies regulate the expression of trophoblast cell adhesion molecules. Fertil Steril. 2002; 77 (4): 805-811.
Ebeling F, Pettersson T, Muukkonen L, Vahtera E and Rasi V. Beta-2-glycoprotein I antibodies in patients with thrombosis. Scand J Clin Lab Invest. 2003; 63 (2): 111-118.
Fischetti F, Durigutto P, Pellis V, Debeus A, Macor P, Bulla R, Bossi F, Ziller F, Sblattero D, Meroni P and Tedesco F. Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood. 2005; 106 (7): 2340-2346.
Fishman P, Falah-Vaknin E, Sredni B, Meroni PL, Rudniki C and Shoenfeld Y. Aspirin modulates interleukin-3 production: additional explanation for the preventive effects of aspirin in antiphospholipid antibody syndrome. J Rheumatol. 1995; 22 (6): 1086-1090.
Fishman P, FaLAh-Vaknin E, Sredni B, Meroni PL, Tincani A, Dicker D and Shoenfeld Y. Aspirin-interleukin-3 interrelationships in patients with anti-phospholipid syndrome. Am J Reprod Immunol. 1996; 35 (2): 80-84.
Fishman P, FaLAh-Vaknine E, Zigelman R, Bakimer R, Sredni B, Djaldetti M and Shoenfeld Y. Prevention of fetal loss in experimental antiphospholipid syndrome by in vivo administration of recombinant interleukin-3. J Clin Invest. 1993; 91 (4): 1834-1837.
Galli M, Luciani D, Bertolini G and Barbui T. Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood. 2003; 101 (5): 1827-1832.
Geva E, Amit A, Lerner-Geva L and Lessing JB. Autoimmunity and reproduction. Fertil Steril. 1997; 67 (4): 599-611.
Girardi G, Redecha P and Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med. 2004; 10 (11): 1222-1226.
Gleicher N. Reproductive failure prior to the onset of clinical autoimmune disease. Rheumatology. 1999; 38 (6): 485-487.
Glinoer D, Soto MF, Bourdoux P, Lejeune B, Delange F, Lemone M, Kinthaert J, Robijn C, Grun JP and de Nayer P. Pregnancy in patients with mild thyroid abnormalities: maternal and neonatal repercussions. J Clin Endocrinol Metab. 1991; 73 (2): 421-427.
Hardy CJ, Palmer BP, Morton SJ, Muir KR and Powell RJ. Pregnancy outcome and family size in systemic lupus erythematosus: a case-control study. Rheumatology (Oxford). 1999; 38 (6): 559-563.
Harel M and Shoenfeld Y. Predicting and preventing autoimmunity, myth or reality? Ann N Y Acad Sci. 2006; 1069 322-345.
Jankowski M, Vreys I, Wittevrongel C, Boon D, Vermylen J, Hoylaerts MF and Arnout J. Thrombogenicity of beta 2-glycoprotein I-dependent antiphospholipid antibodies in a photochemically induced thrombosis model in the hamster. Blood. 2003; 101 (1): 157-162.
Krikun G, Lockwood CJ, Wu XX, Zhou XD, Guller S, Calandri C, Guha A, Nemerson Y and Rand JH. The expression of the pLAental anticoagulant protein, annexin V, by villous trophoblasts: immunolocalization and in vitro regulation. PLAenta. 1994; 15 (6): 601-612.
Lee EY, Lee CK, Lee TH, Chung SM, Kim SH, Cho YS, Yoo B and Moon HB. Does the antibeta2- glycoprotein I antibody provide additional information in patients with thrombosis? Thromb Res. 2003; 111 (1-2): 29-32.
Meroni PL, Borghi MO, Raschi E and Tedesco F. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat Rev Rheumatol. 2011; 7 (6): 330-339.
Meroni PL, di Simone N, Testoni C, D’Asta M, Acaia B and Caruso A. Antiphospholipid antibodies as cause of pregnancy loss. Lupus. 2004; 13 (9): 649-652.
Meroni PL, Tedesco F, Locati M, Vecchi A, Di Simone N, Acaia B, Pierangeli SS and Borghi MO. Anti-phospholipid antibody mediated fetal loss: still an open question from a pathogenic point of view. Lupus. 2010; 19 (4): 453-456.
Nash MJ, Camilleri RS, Kunka S, Mackie IJ, Machin SJ and Cohen H. The anticardiolipin assay is required for sensitive screening for antiphospholipid antibodies. J Thromb Haemost. 2004; 2 (7): 1077-1081.
Park, A.L. in Hughes’ Syndrome (ed. Khamashta, M.A.) Ch. 28 Placental Pathology in antiphospholipid syndrome, 362-374 (Springer-Verlag, London, 2006).
Pengo V, Biasiolo A, Gresele P, Marongiu F, Erba N, Veschi F, Ghirarduzzi A, de Candia E, Montaruli B, Testa S, Barcellona D and Tripodi A. Survey of lupus anticoagulant diagnosis by central evaluation of positive plasma samples. J Thromb Haemost. 2007; 5 (5): 925-930.
Pengo V, Biasiolo A, Pegoraro C, Cucchini U, Noventa F and Iliceto S. Antibody profiles for the diagnosis of antiphospholipid syndrome. Thromb Haemost. 2005; 93 (6): 1147-1152.
Pierangeli SS, Chen PP, Raschi E, Scurati S, Grossi C, Borghi MO, Palomo I, Harris EN and Meroni PL. Antiphospholipid antibodies and the antiphospholipid syndrome: pathogenic mechanisms. Semin Thromb Hemost. 2008; 34 (3): 236-250.
Ramesh S, Morrell CN, Tarango C, Thomas GD, Yuhanna IS, Girardi G, Herz J, Urbanus RT, de Groot PG, Thorpe PE, Salmon JE, Shaul PW and Mineo C. Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via beta2GPI and apoER2. J Clin Invest. 2011; 121 (1): 120-131.
Romay-Penabad Z, Aguilar-Valenzuela R, Urbanus RT, Derksen RH, Pennings MT, Papalardo E, Shilagard T, Vargas G, Hwang Y, de Groot PG and Pierangeli SS. Apolipoprotein E receptor 2 is involved in the thrombotic complications in a murine model of the antiphospholipid syndrome. Blood. 2011; 117 (4): 1408-1414.
Ruffatti A, Tonello M, Del Ross T, Cavazzana A, Grava C, Noventa F, Tona F, Iliceto S and Pengo V. Antibody profile and clinical course in primary antiphospholipid syndrome with pregnancy morbidity. Thromb Haemost. 2006; 96 (3): 337-341.
Ruiz-Irastorza G, Crowther M, Branch W and Khamashta MA. Antiphospholipid syndrome. Lancet. 2010; 376 (9751): 1498-1509.
Singh A, Dantas ZN, Stone SC and Asch RH. Presence of thyroid antibodies in early reproductive failure: biochemical versus clinical pregnancies. Fertil Steril. 1995; 63 (2): 277-281.
Stagnaro-Green A, Roman SH, Cobin RH, el-Harazy E, Alvarez-Marfany M and Davies TF. Detection of at-risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. JAMA. 1990; 264 (11): 1422-1425.
Tincani A, Balestrieri G, Danieli E, Faden D, Lojacono A, Acaia B, Trespidi L, Ventura D and Meroni PL. Pregnancy complications of the antiphospholipid syndrome. Autoimmunity. 2003; 36 (1): 27-32.
Vega-Ostertag ME, Ferrara DE, Romay-Penabad Z, Liu X, Taylor WR, Colden-Stanfield M and Pierangeli SS. Role of p38 mitogen-activated protein kinase in antiphospholipid antibody-mediated thrombosis and endothelial cell activation. J Thromb Haemost. 2007; 5 (9): 1828-1834.
Vlachoyiannopoulos PG, Samarkos M, Sikara M and Tsiligros P. Antiphospholipid antibodies: laboratory and pathogenetic aspects. Crit Rev Clin Lab Sci. 2007; 44 (3): 271- 338.
Wegmann TG. The cytokine basis for cross-talk between the maternal immune and reproductive systems. Curr Opin Immunol. 1989; 2 (5): 765-769.
Yasuda M, Takakuwa K, Tokunaga A and Tanaka K. Prospective studies of the association between anticardiolipin antibody and outcome of pregnancy. Obstet Gynecol. 1995; 86 (4 Pt 1): 555-559.