Iron & Diabetes
A review
Abstract
Both anemia and iron overload are commonly encountered problems in clinical practice. In between is a huge gray area of iron levels, measured by various markers, that fall in the normal reference ranges and are viewed as not a cause for concern. However, to take as an example the risk of acute cardiovascular events in women, the prevalence is very small prior to menopause and then increases to approach the risk level where postmenopausal women have a prevalence similar to adult men. This has been attributed to the very low systemic iron burden in premenopausal women and has encouraged the questioning of the optimum levels in both men and postmenopausal women. This is not a frivolous suggestion since the iron levels, or more properly the iron stores, are easily adjustable by blood donation or phlebotomy and blood iron stores of premenopausal women are easily achieved by adult men, apparently with negligible risk of side effects including anemia. Rather like the argument of those who believe in statins that the lower the LDL the better, a valid concern can be raised as to the appropriate range of iron stores in both adult men and postmenopausal women. Elevated iron stores lead to elevated iron available for redox reactions which act as generators of reactive oxygen species and highly significant inflammation, both of which are associated with a number of chronic diseases having inflammation as a part of their etiology. In this review, the connection between iron overload and diabetes will be discussed.
Introduction
It is well established that humans need a certain level of iron for health, but higher levels are potentially toxic (Kell 2009). From the point of view of evolution, normal stores of iron during reproductive years provided reserves for hemorrhage or periods of severe dietary restrictions or starvation. However there is no homeostatic mechanism for excreting excess iron to maintain a certain level.
Hemochromatosis and thalassaemia involve very high pathological iron levels, but even mildly elevated iron stores over time which are implicated in excessive redox (oxidation-reduction) reactions have been associated with the pathogenesis of cancer, neurodegenerative disorders, atherosclerosis, cardiovascular disease, peripheral artery disease and diabetes (Kell 2009). Iron is mostly sequestered as ferritin, a ubiquitous intracellular protein that both stores iron and releases it in a controlled manner in a safe form. Transferrin also ties up iron but can be saturated. Its function is to transport iron through the blood to the liver, spleen and bone marrow, with the iron in part coming from diet and erythrocyte breakdown. Significant serum levels of non-transferrin bound iron can also be present. It is redox active, and appears to be present even when transferrin saturation is absent (Lee 2006). The blood level of ferritin is the most commonly used marker for the magnitude of iron stores and iron overload.
Elevated ferritin levels are not always a true indicator of iron stores since it is an acute phase reactant. In most studies involving diabetes, this does not appear to be an issue. Mean ferritin levels in the US population study NHANES III were for men about 150 ng/mL, for menstruating women ages 17-49, 25-35 ng/mL, and for menopausal women ages 50-59, 60 ng/mL and for ages > 60, about 90-100 ng/mL (Zacharski 2000). Low levels of stored iron in premenopausal women have been frequently evoked as an explanation for the well known low risk of cardiovascular disease. These numbers raise concerns that the normal male level or the level found in older postmenopausal women may be associated with elevated risk of morbidity or mortality.
Toxicity of Iron
The toxicity of iron is related to its role in producing oxidative damage and the ease with which it is reversibly oxidized and reduced. Ferrous iron catalyzes the production of the highly toxic and reactive hydroxyl radical from hydrogen peroxide, whereas superoxide dismutase serves to equilibrate superoxide and peroxide. Oxidized iron regenerates reduced iron by reacting with superoxide with redox cycling. This results in reactive oxygen species (ROS) responsible for oxidative stress. When these overwhelm the antioxidant capacity, damage occurs which has been related to diseases of aging and inflammation (Brewer 2007). One view is that the dangers of excess iron operate through inadequately liganded (tied up) iron ions. When the ions are tightly liganded they are unreactive but weak ligand formation leaves some reactive free iron. The pathophysiology of iron is thus related to the availability of so-called catalytic iron, or iron that is available to participate in free radical reactions. Due to their weak antioxidant defences the pancreatic β-cells are particularly susceptible to oxidative damage such as can be caused by iron-generated reactive oxygen species (Tiedge 1997). In fact, iron has been used to induce diabetes in animals. Correlations with ferritin levels are an indirect measure of risk from iron-induced oxidative stress, and high ferritin levels correlate with elevated inflammatory cytokine levels (Depalma 2010).
The Association between Iron Levels, Diabetes and the Metabolic Syndrome
The potential role of iron in the pathogenesis of diabetes is suggested by several observations (Rajpathak 2009): (1) Increased incidence of diabetes is seen in patients with diverse causes of iron overload. (2) The reduction in iron load by either chelation or phlebotomy can improve glycemic control or reverse diabetes. (3) Dietary intake of heme iron (e.g. red meat) and concomitant increases in iron stores is associated with the risk of developing diabetes. (4) Insulin sensitivity and insulin secretion are increased by frequent blood donation. The molecular mechanisms are numerous and incompletely understood but include oxidative stress, modulation of adipokines and intracellular singling transduction pathways (Simcox 2013, Swaminathan 2007).
It has been argued that the modest elevations of ferritin observed in diabetes may be a consequence of the disorder rather than a causal factor impacting insulin resistance and β-cell function and apoptosis (Jehn 2007). However, evidence suggests otherwise. Excessive amounts of nontransferrin- bound iron, the form most susceptible to redox activity, are found in diabetic patients with a strong gradient for disease severity (Lee 2006). Furthermore, as will be discussed below, phlebotomy in type 2 diabetes results in improvements in glycemic control and insulin sensitivity which also supports the hypothesis that iron plays a pathogenic role.
Two recently published systematic reviews plus metaanalyses have examined the association of diabetes incidence with ferritin levels. One study (Zhao 2012) reports a meta-analysis of 12 prospective or crosssectional studies which analyzed ferritin levels and involved 4366 type 2 diabetes patients and over 41,000 controls plus four studies that measured heme-iron intake involving 9246 type 2 diabetics and about 180,000 controls. It was found that for the highest vs. the lowest category of ferritin level, the risk of diabetes was increased 66% in prospective studies and 130% in cross-sectional studies. A similar comparison for heme-iron intake yielded a 31% risk increase.
A second study (Bao 2012) examined the association between the risk of diabetes and dietary iron intake in prospective studies. A meta-analysis of five studies gave a pooled relative risk increase of 33% in a comparison of the lowest vs. the highest heme-iron intake. For elevated ferritin levels, they found a 70% increase in relative risk in multivariableadjusted models (seven studies) and a 63% increase in multivariable-adjusted models which included inflammatory markers (five studies). There was no significant association with dietary intake and risk for non-heme or supplemental iron intake, a result consistent with the high bioavailability only of heme iron. Incidentally, another study found a correlation between ferritin levels and the risk of diabetic retinopathy (Canturk 2003).
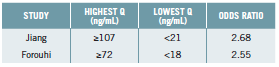
For postmenopausal women (56-62 years of age) who typically have ferritin levels between 70 and 90 ng/dL (Zacharski 2000), data taken from one of the above studies (Bao 2012) for the risk of developing type 2 diabetes are given in the table below according to the highest vs. the lowest ferritin quartiles.
It would seem that for women in this age group, levels similar to the NHANES average already carry significant risk.
A recent review and meta-analysis relates to the above. At issue is the association between red meat intake and risk of developing type 2 diabetes. A 19% increase was found per 100 g/day of red meat and a 51% increase per 50 g/day of processed red meat (Micha 2012).
The metabolic syndrome (MetS) is recognized as a risk factor for diabetes. Population studies find an association between ferritin levels and the risk of MetS in the US (Jehn 2004), Korea (Lee 2011), and Germany (Wrede 2006). Ferritin levels are associated with the MetS in postmenopausal but not premenopausal Korean women (Cho 2011), High levels of ferritin in a Chilean population correlated not only with a three-fold increase in developing MetS but for high levels of oxidative stress indicated by serum markers, there was a 21 fold increase in the development of this syndrome when the highest vs. the lowest quartiles were compared (Leiva 2013).
Clinical use of Phlebotomy to reduce Body Iron Stores
Both blood donation and phlebotomy can dramatically reduce iron stores (Houschyar 2012, Zacharski 2011). Repeated blood letting very efficiently lowers ferritin levels even if the initial values are very high such as seen in hemochromatosis. Furthermore, no induced anemia has been reported. The following studies are of interest:
• In a randomized controlled trial with metabolic syndrome patients, reduction of mean ferritin levels from 183 to 105 ng/mL using phlebotomy resulted in significant reductions in blood glucose, HbA1c, and systolic blood pressure. Changes in blood pressure and the HOMA-IR insulin resistance index correlated with ferritin reduction (Houschyar 2012).
• In a study comparing lacto-ovo vegetarians and meat eaters, the former were found to have mean ferritin levels of 35 ng/mL compared to 72 ng/mL for meats eaters and to have higher insulin sensitivity. When body stores of iron were lowered by phlebotomy in the meat eating group there was a 40% increase in insulin mediated glucose disposal (Hua 2001).
• The effect of phlebotomy on insulin resistance in a group of patients with non-alcoholic fatty liver disease and strongly elevated ferritin levels found a significant reduction in the HOMA-IR from 4.81 to 3.12 when ferritin levels were reduced from 438 to 52 ng/mL (Valenti 2007).
• In a study designed to examine the pathogenesis of diabetes associated with mutations of the hemochromatosis gene, 17 carriers comprising eight patients with diabetes and nine with normal glucose tolerance (NGT) were subjected to phlebotomy and the impact on insulin sensitivity and secretion investigated. Baseline ferritin levels were 942 and 1148 in the NGT and diabetic subjects, respectively. Ferritin targets were ≤ 100 ng/mL or ≤ 50 ng/mL depending of negative or positive evidence for iron deposits in the liver. The target levels were maintained and at 24 months the endpoint parameters measured. In both the NGT and diabetic groups, insulin secretion and insulin sensitivity increased. In the diabetic patients, fasting glucose declined from 137 to 105 mg/dL (7.6 to 5.8 mmol/L), the latter being close to normal (Equitani 2008).
• Earlier studies also found that reduction of iron stores consistently produces an improvement in insulin sensitivity and β-cell function (Fernandez- Real 2002, Fernandez-Real 2005).
Chelation, the Alternative to Phlebotomy or Blood Donation
Advanced glycation end products are thought to play a role in the complications of diabetes and the basic biochemistry involves reactive oxygen species including those attributed to iron activity (Nagai 2012). Oral chelation has been the traditional mainstream approach to iron overload for patients having pathological levels, and several prescription drugs are available. However, iron overloads involved in most of the studies discussed above are nowhere near those encountered in pathological iron overload. Furthermore, while studies, both interventional and observational, suggest target ranges for both men and women, optimum levels are clearly debatable. Chronic low-dose oral chelation therapy may be an important tool for the prevention and treatment of diabetic complications.
If one wishes to use non-pharmaceutical interventions to lower ferritin levels to, for example, to the upper end of the normal range for premenopausal women even if one is male, there are a number of “natural” iron chelators. N-acetyl cysteine is in fact a standard therapy for treating pediatric pathological iron overload even in infants. Green tea extract, curcumin, silymarin, alpha-lipoic acid (or R-lipoic acid) and quercetin all have documented success in iron chelation (Anderson 2012). These chelators also act to eliminate other toxic metals although for mercury it may help to add selenium to N-acetyl cysteine and lipoic acid. Curcumin was recently found to be a very good iron chelator (Jiao 2006). A recent randomized controlled trial demonstrated the effectiveness of curcumin in significantly improving markers of glucose metabolism in diabetics (Chuengsamarn 2012).
The results of the Trial to Access Chelation Therapy (TACT), a randomized, placebo controlled intravenous EDTA chelation trial, recently reported. Included were the results that for diabetics, there was a 39% relative risk reduction and a number needed to treat of eight (median follow-up 55 months) found for the composite primary endpoint of total mortality, recurrent MI, stroke, coronary revascularization or hospitalization for angina (Lamas 2013). EDTA chelation generally results in large increases in urine iron immediately post infusion (Cranton 2001).
The TACT result is particular interesting since intensive glycemic control with multiple drugs and even the addition of insulin fails to impact cardiovascular or total mortality or almost all other complications associated with diabetics (Boussageon 2012, Hemmingsen 2011, Turnbull 2012, Turnbull 2009). The dramatically lowered fasting blood glucose or HbA1c produced by intensive drug therapy may be viewed as successful control and treatment, but engenders false optimism while the pathogenesis of complications relentlessly progresses to ultimately yield clinical manifestations.
Iron Stores Reductions and Diabetic Complications
Studies on humans are limited. A nine-month study among patients with diabetes using deferiprone oral iron chelation reduced ferritin levels from 144 to 59 ng/mL and decreased the mean albumin/creatinine ratio from 187 to 25 mg/L (Rajapurkar 2013). In addition, a study involving the progression of diabetic nephropathy used a polyphenol–enriched, low-iron carbohydrate-restricted diet over four years. There was no significant change in HbA1c, but there was an absolute decrease in the incidence of serum creatinine doubling of 18% and a decrease in mortality or end-stage kidney disease of 18% over four years (number needed to treat over four years for either was six) (Facchini 2003). Iron chelation due to the polyphenols (from red wine, tea and polyphenol enhanced olive oil) was probably partly responsible for reduced ferritin from 325 to 53 ng/mL and may have been a major contributor to these results.
Conclusions
Studies are clearly needed to establish optimum iron stores in both men and women in the context of not only diabetes incidence, progression and complications, but also for all inflammation-related chronic diseases, in particular those associated with aging. Furthermore, large clinical studies are needed to examine the impact of lowering iron stores on the incidence of clinical manifestations of the complications of diabetes, not just markers of glucose metabolism. Can aggressive iron stores reduction, perhaps with severe carbohydrate restriction, cure type 2 diabetes, i.e. eliminate need for any medication? This question should have high priority. There are health issues associated with iron where one of the potentially effective interventions, blood donation, is free with almost no side effects. Iron stores as measured by ferritin can be dramatically lowered in most individuals without inducing anemia.
References:
Anderson, K., 2012. Excess Iron and Brain Degeneration. http://www.lef.org/magazine/mag2012/mar2012_ Excess-Iron-Brain-Degeneration_01.htm.
Bao, W., Rong, Y., Rong, S. and Liu, L. Dietary iron intake, body iron stores, and the risk of type 2 diabetes: a systematic review and meta-analysis. BMC Med 2012; 10: 119.
Boussageon, R., Supper, I., Bejan-Angoulvant, T., Kellou, N., Cucherat, M., Boissel, J.P., Kassai, B., Moreau, A., Gueyffier, F. and Cornu, C. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomised controlled trials. PLoS Med 2012; 9(4): e1001204.
Brewer, G.J. Iron and copper toxicity in diseases of aging, particularly atherosclerosis and Alzheimer’s disease. Exp Biol Med (Maywood. ) 2007; 232(2): 323-335.
Canturk, Z., Cetinarslan, B., Tarkun, I. and Canturk, N.Z. Serum ferritin levels in poorly- and well-controlled diabetes mellitus. Endocr. Res 2003; 29(3): 299-306.
Cho, G.J., Shin, J.H., Yi, K.W., Park, H.T., Kim, T., Hur,J.Y. and Kim,S.H. Serum ferritin levels are associated with metabolic syndrome in postmenopausal women but not in premenopausal women. Menopause. 2011; 18(10): 1120-1124.
Chuengsamarn, S., Rattanamongkolgul, S., Luechapudiporn, R., Phisalaphong, C. and Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012; 35(11): 2121-2127.
Cranton, E.M., 2001. A Textbook on EDTA Chelation Therapy. Hamptom Roads Publishing Co., Inc., Charlottesville, VA.
Depalma, R.G., Hayes, V.W., Chow, B.K., Shamayeva, G., May, P.E. and Zacharski, L.R. Ferritin levels, inflammatory biomarkers, and mortality in peripheral arterial disease: a substudy of the Iron (Fe) and Atherosclerosis Study (FeAST) Trial. J Vasc Surg 2010; 51(6): 1498-1503.
Equitani, F., Fernandez-Real, J.M., Menichella, G., Koch, M., Calvani, M., Nobili, V., Mingrone, G. and Manco, M. Bloodletting ameliorates insulin sensitivity and secretion in parallel to reducing liver iron in carriers of HFE gene mutations. Diabetes Care 2008; 31(1): 3-8.
Facchini, F.S. and Saylor, K.L. A low-iron-available, polyphenol-enriched, carbohydrate-restricted diet to slow progression of diabetic nephropathy. Diabetes 2003; 52(5): 1204-1209.
Fernandez-Real, J.M., Lopez-Bermejo, A. and Ricart, W. Iron stores, blood donation, and insulin sensitivity and secretion. Clin Chem 2005; 51(7): 1201-1205.
Fernandez-Real, J.M., Penarroja, G., Castro, A., Garcia-Bragado, F., Hernandez-Aguado, I. and Ricart, W. Blood letting in high-ferritin type 2 diabetes: effects on insulin sensitivity and beta-cell function. Diabetes 2002; 51(4): 1000-1004.
Hemmingsen, B., Lund, S.S., Gluud, C., Vaag, A., Almdal, T., Hemmingsen, C. and Wetterslev, J. Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ 2011; 343: d6898.
Houschyar, K.S., Ludtke, R., Dobos, G.J., Kalus, U., Broecker- Preuss, M., Rampp, T., Brinkhaus, B. and Michalsen, A. Effects of phlebotomy-induced reduction of body iron stores on metabolic syndrome: results from a randomized clinical trial. BMC Med 2012; 10: 54.
Hua, N.W., Stoohs, R.A. and Facchini, F.S. Low iron status and enhanced insulin sensitivity in lacto-ovo vegetarians. Br J Nutr 2001; 86(4): 515-519.
Jehn,M., Clark, J.M. and Guallar, E. Serum ferritin and risk of the metabolic syndrome in U.S. adults. Diabetes Care 2004; 27(10): 2422-2428.
Jehn,M.L., Guallar, E., Clark, J.M., Couper, D., Duncan, B.B., Ballantyne, C.M., Hoogeveen, R.C., Harris, Z.L. and Pankow, J.S. A prospective study of plasma ferritin level and incident diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol 2007; 165(9): 1047-1054.
Jiao,Y., Wilkinson, J., Christine, P.E., Buss, J.L., Wang, W., Planalp, R., Torti, F.M. and Torti, S.V. Iron chelation in the biological activity of curcumin. Free Radic Biol Med 2006; 40(7): 1152-1160.
Kell, D.B. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genomics 2009; 2: 2.
Lamas, G.A., Goertz, C., Boineau, R., Mark, D.B., Rozema, T., Nahin, R.L., Lindblad, L., Lewis, E.F., Drisko, J. and Lee, K.L. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA 2013; 309(12): 1241-1250.
Lee, B.K., Kim, Y. and Kim, Y.I. Association of serum ferritin with metabolic syndrome and diabetes mellitus in the South Korean general population according to the Korean National Health and Nutrition Examination Survey 2008. Metabolism 2011; 60(10): 1416-1424.
Lee, D.H., Liu, D.Y., Jacobs, D.R., Jr., Shin, H.R., Song, K., Lee, I.K., Kim, B. and Hider, R.C. Common presence of nontransferrin- bound iron among patients with type 2 diabetes. Diabetes Care 2006; 29(5): 1090-1095.
Leiva, E., Mujica, V., Sepulveda, P., Guzman, L., Nunez, S., Orrego, R., Palomo, I., Andrews, M. and Arredondo, M.A. High levels of iron status and oxidative stress in patients with metabolic syndrome. Biol Trace Elem. Res 2013; 151(1): 1-8.
Micha, R., Michas, G. and Mozaffarian, D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes–an updated review of the evidence. Curr Atheroscler. Rep 2012; 14(6): 515-524.
Nagai, R., Murray, D.B., Metz, T.O. and Baynes, J.W. Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes 2012; 61(3): 549-559.
Rajapurkar, M.M., Hegde, U., Bhattacharya, A., Alam, M.G. and Shah, S.V. Effect of deferiprone, an oral iron chelator, in diabetic and non-diabetic glomerular disease. Toxicol Mech Methods 2013; 23(1): 5-10.
Rajpathak, S.N., Crandall, J.P., Wylie-Rosett, J., Kabat, G.C., Rohan, T.E. and Hu, F.B. The role of iron in type 2 diabetes in humans. Biochim Biophys Acta 2009; 1790(7): 671-681.
Simcox, J.A. and McClain, D.A. Iron and diabetes risk. Cell Metab 2013; 17(3): 329-341.
Swaminathan, S., Fonseca, V.A., Alam, M.G. and Shah, S.V. The role of iron in diabetes and its complications. Diabetes Care 2007; 30(7): 1926-1933.
Tiedge, M., Lortz, S., Drinkgern, J. and Lenzen, S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997; 46(11): 1733-1742.
Turnbull, F. and Zoungas, S. Intensive glucose-lowering therapy in people with type 2 diabetes: what do we learn from a new meta-analysis of randomised controlled trials? Evid. Based. Med 2012; 17(3): 98-99.
Turnbull, F.M., Abraira, C., Anderson, R.J., Byington, R.P., Chalmers, J.P., Duckworth, W.C., Evans, G.W., Gerstein, H.C., Holman, R.R., Moritz, T.E., Neal, B.C., Ninomiya,T., Patel, A.A., Paul, S.K., Travert, F. and Woodward, M. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009; 52(11): 2288-2298.
Valenti, L., Fracanzani, A.L., Dongiovanni, P., Bugianesi, E., Marchesini, G., Manzini, P., Vanni, E. and Fargion, S. Iron depletion by phlebotomy improves insulin resistance in patients with nonalcoholic fatty liver disease and hyperferritinemia: evidence from a case-control study. Am J Gastroenterol 2007; 102(6): 1251-1258.
Wrede, C.E., Buettner, R., Bollheimer, L.C., Scholmerich, J., Palitzsch, K.D. and Hellerbrand, C. Association between serum ferritin and the insulin resistance syndrome in a representative population. Eur J Endocrinol 2006; 154(2): 333-340.
Zacharski, L.R., Ornstein, D.L., Woloshin, S. and Schwartz, L.M. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J 2000; 140(1): 98-104.
Zacharski, L.R., Shamayeva, G. and Chow, B.K. Effect of controlled reduction of body iron stores on clinical outcomes in peripheral arterial disease. Am Heart J 2011; 162(5): 949-957.
Zhao, Z., Li, S., Liu, G., Yan, F., Ma, X., Huang, Z. and Tian, H. Body iron stores and heme-iron intake in relation to risk of type 2 diabetes: a systematic review and meta-analysis. PLoS One 2012; 7(7): e41641.