HPA Axis Dysregulation and Human Health
What is the evidence?
Regulation of hypothalamus-pituitary-adrenal (HPA) axis activity has far reaching implications for many areas of human health. Abnormalities in HPA axis reactivity and secretion of its primary effector, cortisol, have been observed in various autoimmune diseases, chronic fatigue syndrome (CFS) and fibromyalgia (FM), as well as in patients with major depression and cancer (Brivio 2010, Chrousos 1995, Nussey 2001, Romer 2009). Cortisol exerts widespread anti-inflammatory, immunosuppressive, catabolic, hyperglycemic, and anti-reproductive effects that may modulate these disease processes; strategies that modulate cortisol activity either through direct hormone replacement, or herbal/ nutritional/ lifestyle strategies, may be useful as therapy for such conditions (Chrousos 1995). This article reviews determinants of the HPA stress response, the concept of hypoadrenalism/ adrenal fatigue, and existing human trials of low-dose hydrocortisone replacement. HPA axis function in specific conditions, adrenal function testing, and nutritional/ herbal interventions strategies are to be discussed in future articles.
HPA Axis Physiology
Activation of the HPA axis in response to a perceived stressor begins with the secretion of corticotropin releasing hormone (CRH) and arginine-vasopressin (AVP) from the paraventricular nucleus (PVN) of the hypothalamus (Smith 2006, Tsigos 2002). This triggers release of adrenocorticotropic hormone (ACTH) from the anterior pituitary, which in turn stimulates the secretion of glucocorticoids, notably cortisol, by the adrenal cortex. Cortisol acts through the ubiquitous distribution of glucocorticoid receptor (GR) throughout the body, altering cellular gene expression through activation of glucocorticoid response elements (Chrousos 1995). Inhibition of the HPA axis is by negative feedback of cortisol on hypothalamic and pituitary centers, thus limiting the duration of tissue exposure to the deleterious effects of chronic elevated cortisol (Nussey 2001).
Stressors
Stressors that drive CRH/ cortisol secretion can be categorized as physical stressors such as exertion, trauma, and lack of sleep; psychological stressors; and biochemical stressors such as hypoglycemia and inflammation (Balbo 2010). Sleep deprivation or irregularity is an often overlooked contributor to a patient’s overall stress load. Sleep onset modestly inhibits cortisol secretion, while awakenings are accompanied by increased secretion, an effect that is likely mediated in part by melatonin, the diurnal counterpart to cortisol (Balbo 2010). Chapotot demonstrated that plasma cortisol level, secretory rate, and pulse amplitude were all significantly increased in healthy young men after a night of sleep deprivation compared to regular nocturnal sleep (p<0.05) (2001). Sleep phase shift like that which occurs during travel across time zones or with irregular hours of sleep also induces dramatic increases in cortisol (Balbo 2010).
Inflammatory mediators such as TNF-α, IL-1, and IL-6 have also been shown to activate the HPA axis (Tsigos 2002). These cytokines, particularly IL-6, signal the HPA axis at multiple levels (hypothalamus, pituitary, and adrenal glands) to secrete more glucocorticoids in order to control inflammation (Chrousos 1995, Mastorakos 1995). Tsigos showed that administration of IL-6, a key mediator of inflammation, resulted in acutely elevated plasma ACTH and elevation in cortisol lasting for over four hours in healthy subjects (1997). Mastorakos found that IL-6 administration increased ACTH and cortisol levels in cancer patients (1993). Interestingly, Vgontzas showed that sleep deprivation in otherwise healthy subjects induced significantly increased daytime secretion of IL-6, which may represent an indirect mechanism by which sleep loss induces cortisol (1999).
Rapidly fluctuating blood glucose levels represent another unsuspected source of biochemical stress. Hypoglycemia requires adrenal compensation, triggering cortisol mediated liver gluconeogenesis in order to restore normal glucose levels (Chen 2010). It is interesting to note that a feeding response is also commonly observed, such that cortisol tends to rise modestly after meals, particularly at noon (Lemmens 2011, Vicennati 2002). Although there is conflicting evidence, it seems that intake of carbohydrates and simple sugar results in a higher post meal cortisol response compared to water, fats, and protein (Martens 2010), while animal studies suggest that high protein and high fat foods may reduce post meal cortisol (Lacroix 2004). This makes sense in light of the fact that consumption of fats and protein results in a smoother, more even post-prandial blood glucose profile, requiring less compensation by the adrenal glands. A corollary of this is that functional hypoglycemia may therefore also be an indication of hypoadrenalism (Gaby 2010).
Obesity has been associated with greater 24 hour cortisol secretion (Vicennati 2002), which may be due in part to the inflammatory milieu created by abdominal adiposity, with elevated CRP etc, however obesity is also characterized by HPA axis hyperresponsiveness to neuroendocrine stimuli (Vicennati 2000, 2004). Vincennati conducted a study of HPA stimulation and suppression through the administration of arginine-vasopressin (AVP, an HPA stimulant produced by the hypothalamus) and alprazolam (a short acting benzodiazepine and centrally acting HPA inhibitor used to treat anxiety) in women (2004). Overweight/ obese women showed higher ACTH and cortisol responses to the AVP stimulation test, and significantly greater inhibition after alprazolam compared to non-overweight controls (Vicennati 2004). Alprazolam also decreased 24h-urinary free cortisol excretion by approximately 50% in the overweight/obese subjects, but not in controls.
Other endocrine axes also interact with cortisol and the HPA-axis. In women, an age-related decrease in estradiol is thought to decrease the responsiveness of the HPA axis to stimulatory cytokines, based on evidence in animal models of ovarian failure (Straub 2000, Xia-Zhang 1995), and estrogen replacement therapy in menopausal women has been shown to suppresses the HPA axis response to emotional stress (Carrasco 2003, Lindheim 1992). Tsigos reports that activation of the HPA axis is associated with decreased production of TSH and decreased peripheral conversion of T4 to the more biologically active T3 (as seen in ‘‘euthyroid sick’’ syndrome), which may serve to conserve energy during stress (2002). In animal models, induction of the stress response (ACTH secretion) has been shown to decrease circulating levels of T4 and T3 (Helmreich 2005).
Cortisol synthesis
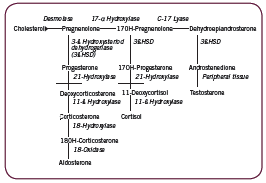
The adrenal cortex is responsible for three major pathways/ products: the mineralocorticoid pathway leading to production of aldosterone; the androgen/ estrogen pathway producing DHEA, testosterone, and estradiol; and the glucocorticoid pathway leading to production of cortisol. These are depicted in Figure 1. Two key enzymes convert intermediaries to cortisol: 21ß-hydroxylase converts 17-hydroxy-progesterone to 11- ßdeoxycortisol, which is then converted to cortisol by 11ß-hydroxylase. Measurement of serum 17-hydroxy-progesterone (17-OH-P) level is often used to assess the activity of these enzymes; in the event of an enzyme deficiency, as in non-classical congenitial adrenal hyperplasia (NCAH) for example, 17-OH-P will accumulate in the pathway (Kelestimur 2006). Negative (eg. catabolic) effects of cortisol are countered in part by the adrenal androgen DHEA (Straub 2000).
Cortisol rhythm
Cortisol secretion is pulsatile, diurnal, and stress-responsive (Balbo 2010). ACTH and cortisol levels peak in the morning (acrophase) just prior to waking, with gradual decline throughout the rest of the day, and an extended period of low levels around midnight (nadir), followed by a rapid rise during the second half of the night (Balbo 2010). Control of diurnal cortisol secretion is thought to be under direct neural control via a multisynaptic pathway from the pineal gland (site of melatonin synthesis), as well as through an intrinsic circadian oscillator in the adrenal gland itself that can be directly modulated by melatonin (Balbo 2010).
The Evolution of HPA and Disease
Seyle’s now-famous General Adaptation Syndrome (GAS) describes three phases of HPA activity in response to stress; the Alarm Reaction, Resistance, and Exhaustion. His 1950 paper describes the biology of each phase in surprising detail. In brief, during the first stage the adrenal glands release epinephrine and glucocorticoids to help restore homeostasis; when homeostasis is restored, adaptation is maintained through sustained secretion of glucocorticoids; when resistance to the stressor can no longer be maintained, exhaustion ensues, accompanied by the onset of disease. This has usually been thought of as the downregulation over time of adrenal gland responsiveness to ACTH/ stressors, or decreased adrenal reserve, resulting in inadequate cortisol secretion to meet physiological requirements and consequent loss of anti-inflammatory control (Gaby 2011, Jefferies 2004). Seyle wrote about “diseases of adaptation” (1950), providing a detailed list of chronic inflammatory diseases, including allergies, asthma, eczema, psoriasis, lupus, rheumatoid arthritis, ulcerative colitis, and chronic urticaria, which may be one of the earliest medical descriptions of what is now considered by naturopathic doctors as “adrenal fatigue” or mild hypoadrenalism.
Although glucocorticoid therapy has since evolved such that standard use involves large pharmacological doses for limited periods of time, William Jefferies pioneered the use of physiologic doses of cortisol as long term therapy of many conditions (2004). Jefferies distinguishes between 1) replacement dosage (~35-40 mg/d) such as that used in adrenalectomized patients or in Addison’s disease; 2) supra-replacement dosage (>40mg/d) or pharmacological doses; and 3) sub-replacement dosage, the dose he utilizes as physiologic cortisol therapy in patients with intact adrenals: hydrocortisone 20mg/d in four divided doses, with food to avoid gastrointestinal side effects such as hyperacidity (2004). In his book, Safe Uses of Cortisol, Jefferies reports cases of many patients with diverse clinical presentations from his own practice who have benefited from physiologic doses of cortisol: rheumatoid arthritis and autoimmune disease, allergies, infertility and recurrent miscarriage, viral infections, functional hypoglycemia, thyroid conditions, and chronic fatigue syndrome (Jefferies 2004).
Jefferies explains that although it is low dose, the physiologic use of cortisol has not historically been considered as a therapeutic option because of the assumption that it carries the same safety concerns as pharmacological doses (eg. diabetes, osteoporosis, etc). He emphasizes the importance of four divided doses (roughly q6h) rather than twice daily “because of evidence that normal blood levels and some metabolic effects … do not last longer than eight hours” (2004). This maintains a smoother cortisol curve without sharp peaks and troughs throughout the day, which more closely mimics physiological secretion. A retrospective chart analysis by Howlett lends support to this dosing schedule: using data from 130 patients and 174 3-point serum cortisol curves, Howlett found that TID dosing achieved “optimal replacement” more frequently than BID dosing, 60% versus 15% of cases (p<0.001) (1997). Optimal replacement was defined as the dose that achieved a urinary free cortisol and 0900h serum cortisol within the normal range to avoid over-replacement, while maintaining 1230h and 1730h cortisol above 50nmol/L, and ideally above 100 nmol/L to avoid under-replacement (1997).
In his text, Nutritional Medicine, Alan Gaby discusses mild hypoadrenalism or adrenocortical insufficiency, differentiating it from the severe, autoimmune hypoadrenalism of Addison’s disease (2011). He lists characteristic symptoms as including fatigue, weakness, anorexia, nausea, vomiting, weight loss, salt craving, hypotension or orthostatic hypotension, hypoglycemia, hyperpigmentation of the skin, decreased body hair in women, and poor tolerance to stress or exertion. Gaby notes that the inability of a clinically hypothyroid patient to tolerate even low doses of thyroid hormone may indicate hypoadrenalism. Gaby also describes low dose hydrocortisone therapy as a treatment option for hypoadrenalism and chronic fatigue syndrome, corroborating Jefferies’ experience (2011). He relates that he uses the lowest effective dose of hydrocortisone, sometimes lower than 5mg QID, and has noted improvement at doses as low as 1mg TID.
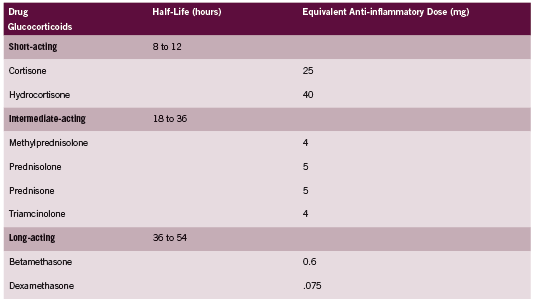
Hydrocortisone is the glucocorticoid of choice for treatment of hypoadrenalism because it is the biochemical equivalent to endogenous cortisol. Other corticosteroids are often more potent in their activity, and as discussed above are dosed very differently. A comparison of glucocorticoids adapted from the Compendium of Pharmaceuticals and Specialties (CPS 2011) is provided in Table 1. According to this table, Prednisone is approximately four times as potent as hydrocortisone.
Human Trials of Hydrocortisone Therapy: CFS and PTSD Chronic Fatigue Syndrome
It has been noted by many authors that CFS resembles Addison’s in its symptomotology (Cleare 2001, Gaby 2011). HPA abnormalities have been documented at many levels of the axis in CFS, but not all with equal consistency (Chrousos 1995, Cleare 2001, Gaab 2002, Jerjes 2007, Papadopoulos 2009, Scott 2000). Cleare reviews HPA axis activity in CFS, stating that “tests of negative feedback using dexamethasone support [the] hypersuppression of cortisol and negative feedback…with over half the studies [also] suggesting lowered basal cortisol and/ or blunted HPA axis responses” (2004). A detailed analysis is beyond the scope of this paper, however three controlled human trials of low dose hydrocortisone therapy conducted in patients with CFS are discussed below.
Cleare found benefit from hydrocortisone in a randomized placebo controlled crossover trial of 32 CFS patients without comorbid psychiatric disorder (1999, 2001). Two dosages investigated: either 5mg or 10mg, taken as a single daily dose with breakfast for 28 days. Both dosages had comparable effects on fatigue scores (p=0.90). In the collective treatment group fatigue score dropped 7.2 points, compared to 3.3 points in the placebo group, a mean difference of 4.5 points (p<0.009). Mean disability score also decreased with treatment but not placebo. Fatigue scores normalized in nine (28%) of the hydrocortisone group compared to two (9%) of the placebo group (p=0.05). Importantly, insulin stress testing showed that these doses of hydrocortisone did not induce adrenal suppression. Reported side effects in three patients on hydrocortisone included: worsening of acne, nervousness, and improvement of eczema.
In contrast, RCTs by McKenzie and Blockmans found no clinically significant benefits, albeit at doses and schedules that were shown to induce adrenal suppression: 25-35mg hydrocortisone BID and a combination of 5mg hydrocortisone + 20mg fludrocortisone daily (Blockmans 2003, McKenzie 1998). McKenzie did find that a greater percentage (53% vs 29%; P=.04) recorded an improvement of five or more points in Wellness score, and a higher average improvement in Wellness score on more days compared to placebo recipients (P<.001) (1998). A lack of correlation between the magnitude of impaired basal/ stimulated serum cortisol levels and disease severity in this study, yet small improvements with hydrocortisone therapy suggests the possibility of peripheral glucocorticoid resistance (Chrousos 1995, McKenzie 1998). Blockmans notes that CFS is a heterogeneous disorder, and treatment with low-dose steroids may only be effective for a certain subset of patients (2003).
Although hydrocortisone for treatment of fibromyalgia has not been investigated, patients with this condition also display HPA axis dysfunction, with lowered salivary cortisol, urinary cortisol excretion and more pronounced suppression of cortisol compared with controls on low dose dexamethasone testing (Izquierdo- Alvarez 2008, Riva 2010, Wingenfeld 2007).
Post Traumatic Stress Disorder (PTSD)
Aerni conducted a small randomized, double blind, placebo controlled cross over trial of three patients with PTSD (2004). Low dose hydrocortisone 10mg doses either as a single daily dose in one patient and as two divided doses in the other two patients or placebo was given for one month. Results showed that there was a significant treatment effect in all the patients, with “cortisolrelated reductions of at least 38% in one of the daily rated symptoms of traumatic memories, as assessed by self-administered rating scales” (Aerni 2004). Additionally, the clinician-Administered PTSD Scale ratings assessed after each month showed cortisolrelated improvements for reexperiencing symptoms, and in one patient, for avoidance symptoms also, but there was no impact on hyperarousal symptoms.
Conclusion
The HPA axis is activated in response to physical, psychological, and biochemical stresses. Mild hypoadrenalism is thought to contribute to the onset of a spectrum of chronic inflammatory conditions. Drs Jefferies and Gaby have pioneered the use of physiological doses of hydrocortisone as therapy for autoimmune disease and other conditions such as chronic fatigue syndrome. Although limited, human trials of low dose hydrocortisone suggest that this therapy may be of benefit to some patients with this condition, with one trial suggesting benefit in approximately 1/3 of patients. There is a need for better understanding of testing methods and natural strategies that can be used to treat conditions characterized by mild hypoadrenalism.
References:
Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, Nitsch RM, Schnyder U, de Quervain DJ. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry. 2004 Aug;161(8):1488-90.
Balbo M, Leproult R, Van Cauter E. Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. Int J Endocrinol. 2010;2010:759234.
Blockmans D, Persoons P, Van Houdenhove B, Lejeune M, Bobbaers H. Combination therapy with hydrocortisone and fludrocortisone does not improve symptoms in chronic fatigue syndrome: a randomized, placebo-controlled, double-blind, crossover study. Am J Med. 2003 Jun 15;114(9):736-41.
Brivio F, Fumagalli L, Fumagalli G, Pescia S, Brivio R, Di Fede G, Rovelli F, Lissoni P. Synchronization of cortisol circadian rhythm by the pineal hormone melatonin in untreatable metastatic solid tumor patients and its possible prognostic significance on tumor progression. In Vivo. 2010 Mar-Apr;24(2):239-41.
Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003 Feb 28;463(1-3):235-72.
Chapotot F, Buguet A, Gronfier C, Brandenberger G. Hypothalamo-pituitary-adrenal axis activity is related to the level of central arousal: effect of sleep deprivation on the association of high-frequency waking electroencephalogram with cortisol release. Neuroendocrinology. 2001 May;73(5):312-21.
Chen CL, Willis BA, Mooney L, Ong GK, Lim CN, Lowe SL, Tauscher-Wisniewski S, Cutler GB Jr, Wiss SD. Cortisol response to individualised graded insulin infusions: a reproducible biomarker for CNS compounds inhibiting HPA activation. Br J Clin Pharmacol. 2010 Dec;70(6):886-94.
Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995 May 18;332(20):1351-62.
Cleare AJ. The HPA axis and the genesis of chronic fatigue syndrome. Trends Endocrinol Metab. 2004 Mar;15(2):55-9.
Cleare AJ, Miell J, Heap E, Sookdeo S, Young L, Malhi GS, O’Keane V. Hypothalamopituitary- adrenal axis dysfunction in chronic fatigue syndrome, and the effects of lowdose hydrocortisone therapy. J Clin Endocrinol Metab. 2001 Aug;86(8):3545-54.
Cleare AJ, Heap E, Malhi GS, Wessely S, O’Keane V, Miell J. Low-dose hydrocortisone in chronic fatigue syndrome: a randomised crossover trial. Lancet. 1999 Feb 6;353(9151):455-8.
Compendium of Pharmaceuticals and Specialties. Corticosteroids: Systemic (CphA Monograph). Updated 2011. www-e-therapeutics-ca. Accessed 10 May 2011.
Gaab J, Hüster D, Peisen R, Engert V, Schad T, Schürmeyer TH, Ehlert U. Low-dose dexamethasone suppression test in chronic fatigue syndrome and health. Psychosom Med. 2002 Mar-Apr;64(2):311-8.
Gaby A. Nutritional Medicine. Concord: Fritz Perlberg Publishing, 2011.
Groves RW, Toms GC, Houghton BJ, Monson JP. Corticosteroid replacement therapy: twice or thrice daily? J R Soc Med. 1988 Sep;81(9):514-6.
Helmreich DL, Parfitt DB, Lu XY, Akil H, Watson SJ. Relation between the hypothalamic-pituitary-thyroid (HPT) axis and the hypothalamic-pituitary-adrenal (HPA) axis during repeated stress. Neuroendocrinology. 2005;81(3):183-92.
Hori H, Teraishi T, Sasayama D, Ozeki Y, Matsuo J, Kawamoto Y, Kinoshita Y, Hattori K, Higuchi T, Kunugi H. Poor sleep is associated with exaggerated cortisol response to the combined dexamethasone/CRH test in a non-clinical population. J Psychiatr Res. 2011 Apr 26. [Epub ahead of print]
Howlett TA. An assessment of optimal hydrocortisone replacement therapy. Clin Endocrinol (Oxf). 1997 Mar;46(3):263-8.
Izquierdo-Alvarez S, Bocos-Terraz JP, Bancalero-Flores JL, Pavón-Romero L, Serrano-Ostariz E, de Miquel CA. Is there an association between fibromyalgia and below-normal levels of urinary cortisol? BMC Res Notes. 2008 Dec 22;1:134.
Jefferies W. Safe Uses of Cortisol, Third Edition. Springfield: Charles C Thomas, 2004.
Jerjes WK, Taylor NF, Wood PJ, Cleare AJ. Enhanced feedback sensitivity to prednisolone in chronic fatigue syndrome. Psychoneuroendocrinology. 2007 Feb;32(2):192-8.
Kelestimur F. Non-classic congenital adrenal hyperplasia. Pediatr Endocrinol Rev. 2006 Aug;3 Suppl 3:451-4.
Lacroix M, Gaudichon C, Martin A, Morens C, Mathé V, Tomé D, Huneau JF. A longterm high-protein diet markedly reduces adipose tissue without major side effects in Wistar male rats. Am J Physiol Regul Integr Comp Physiol. 2004 Oct;287(4):R934-42.
Lemmens SG, Born JM, Martens EA, Martens MJ, Westerterp-Plantenga MS. Influence of consumption of a high-protein vs. high-carbohydrate meal on the physiological cortisol and psychological mood response in men and women. PLoS One. 2011 Feb 3;6(2):e16826.
Lindheim SR, Legro RS, Bernstein L, Stanczyk FZ, Vijod MA, Presser SC, Lobo RA. Behavioral stress responses in premenopausal and postmenopausal women and the effects of estrogen. Am J Obstet Gynecol. 1992 Dec;167(6):1831-6.
Martens MJ, Rutters F, Lemmens SG, Born JM, Westerterp-Plantenga MS. Effects of single macronutrients on serum cortisol concentrations in normal weight men. Physiol Behav. 2010 Dec 2;101(5):563-7.
Mastorakos G, Magiakou MA, Chrousos GP. Effects of the immune/inflammatory reaction on the hypothalamic-pituitary-adrenal axis. Ann N Y Acad Sci. 1995 Dec 29;771:438-48.
Mastorakos G, Chrousos GP, Weber JS. Recombinant interleukin-6 activates the hypothalamic-pituitary-adrenal axis in humans. J Clin Endocrinol Metab. 1993 Dec;77(6):1690-4.
McKenzie R, O’Fallon A, Dale J, Demitrack M, Sharma G, Deloria M, Garcia- Borreguero D, Blackwelder W, Straus SE. Low-dose hydrocortisone for treatment of chronic fatigue syndrome: a randomized controlled trial. JAMA. 1998 Sep 23- 30;280(12):1061-6.
Nussey S, Whitehead S. Endocrinology: An Integrated Approach. Chapter 4: The Adrenal Gland. Feedback control of glucocorticoids. BIOS Scientific Publishers Limited, 2001. Available at NCBI: http://www.ncbi.nlm.nih.gov/books/NBK26/ Accessed 27 May 2011.
Riva R, Mork PJ, Westgaard RH, Rø M, Lundberg U. Fibromyalgia syndrome is associated with hypocortisolism. Int J Behav Med. 2010 Sep;17(3):223-33.
Römer B, Lewicka S, Kopf D, Lederbogen F, Hamann B, Gilles M, Schilling C, Onken V, Frankhauser P, Deuschle M. Cortisol metabolism in depressed patients and healthy controls. Neuroendocrinology. 2009;90(3):301-6.
Scott LV, Svec F, Dinan T. A preliminary study of dehydroepiandrosterone response to low-dose ACTH in chronic fatigue syndrome and in healthy subjects. Psychiatry Res. 2000 Dec 4;97(1):21-8.
Selye H. Stress and the general adaptation syndrome. Br Med J. 1950 Jun 17;1(4667):1383-92.
Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8(4):383-95.
Straub RH, Schölmerich J, Zietz B. Replacement therapy with DHEA plus corticosteroids in patients with chronic inflammatory diseases–substitutes of adrenal and sex hormones. Z Rheumatol. 2000;59 Suppl 2:II/108-18.
Tsigos C, Papanicolaou DA, Defensor R, Mitsiadis CS, Kyrou I, Chrousos GP. Dose effects of recombinant human interleukin-6 on pituitary hormone secretion and energy expenditure. Neuroendocrinology. 1997 Jul;66(1):54-62.
Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002 Oct;53(4):865-71.
Vicennati V, Pasquali R. Abnormalities of the hypothalamic-pituitary-adrenal axis in nondepressed women with abdominal obesity and relations with insulin resistance: evidence for a central and a peripheral alteration. J Clin Endocrinol Metab. 2000 Nov;85(11):4093-8.
Vicennati V, Ceroni L, Gagliardi L, Gambineri A, Pasquali R. Comment: response of the hypothalamic-pituitary-adrenocortical axis to high-protein/fat and highcarbohydrate meals in women with different obesity phenotypes. J Clin Endocrinol Metab. 2002 Aug;87(8):3984-8.
Vicennati V, Ceroni L, Gagliardi L, Pagotto U, Gambineri A, Genghini S, Pasquali R. Response of the hypothalamic-pituitary-adrenal axis to small dose arginine-vasopressin and daily urinary free cortisol before and after alprazolam pre-treatment differs in obesity. J Endocrinol Invest. 2004 Jun;27(6):541-7.
Vgontzas AN, Mastorakos G, Bixler EO, Kales A, Gold PW, Chrousos GP. Sleep deprivation effects on the activity of the hypothalamic-pituitary-adrenal and growth axes: potential clinical implications. Clin Endocrinol (Oxf). 1999 Aug;51(2):205-15.
Wingenfeld K, Wagner D, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim C. The low-dose dexamethasone suppression test in fibromyalgia. J Psychosom Res. 2007 Jan;62(1):85-91.
Xia-Zhang L, Xiao E, Ferin M. A 5-day estradiol therapy, in amounts reproducing concentrations of the early-mid follicular phase, prevents the activation of the hypothalamo-pituitary-adrenal axis by interleukin-1 alpha in the ovariectomized rhesus monkey. J Neuroendocrinol. 1995 May;7(5):387-92.