Effectiveness and safety of the homeopathic preparation Pasconal® Nerventropfen in patients with nervous disorders
ABSTRACT
Objective:
We investigated the safety and effectiveness of a homoeopathic combination preparation in patients suffering from nervousness and sleep disorders.
Methods:
In this observational study, 325 patients suffering from nervous disturbances and sleep disorders were treated for about four weeks with a homeopathic combination preparation containing Avena sativa, Valeriana, Ignatia and Tarantula. The effectiveness and safety was assessed by a questionnaire filled out by the therapists.
Results:
Patients without concomitant medication and therapy duration over four weeks responded better to the therapy with the homeopathic preparation. A clear reduction in the symptom severity could be observed for all 12 analyzed symptoms. In patients who did not receive concomitant medication due to the inclusion diagnosis, a comparatively more positive and significant (p < 0.05) effect on the symptoms nervousness/restlessness and irritability/eccentricity was observed. The effect of PASCONAL® NERVENTROPFEN on five individual symptoms (nervousness/restlessness, irritability/eccentricity, sleep disorders, fitful sleep, and hyperactivity) was more pronounced in patients who were treated for four weeks or more than in patients with a shorter treatment period. All p-values for comparison between the two subgroups were < 0.05. The therapy was well-tolerated.
Conclusion:
This observational study indicates that the homoeopathic combination preparation is an effective and well-tolerated alternative to pharmaceuticals for the treatment of nervous disturbances and sleep disorders.
Keywords:
nervousness, sleep disorders, homeopathic combination preparation, Avena sativa, Valeriana, Ignatia, Tarantula
INTRODUCTION
Stress dominates the lives of many people, frequently leading to undesirable health consequences, including nervousness, tension and sleep disorders. Stress often arises as a response to current circumstances of life, such as job-related, school, or private stress; rising workload or academic demands; overstimulation; inability to relax; burdening living conditions; and personal disappointments or losses. Psychiatric disorders such as sleep disturbances, psychosomatic complaints, states of anxiety and depression occur far more often than is widely believed (Kraft 2007).
The spectrum of interventions for nervousness, restlessness, and sleep disturbances is broad, and treatments range from benzodiazepines and anxiolytics to relaxations techniques (Hankey 2006) and homoeopathic preparations (Kumar 2005). Traditional allopathic medicine is the most common mode of treatment for stress-related conditions but because side effects, interactions, and the potential for pharmaceutical dependence do not allow long-term treatment (Rosenberg 2006, Tan 2010), alternativetherapies are increasingly employed (Barnes 2004, Schneider 2004). Positive results have been reported for the treatment of sleep disorders (Coholic 2005, Waldschutz 2008), mild nervous disorders (Meerschaut 2007), and mental trauma (Ventegodt 2006) with complementary therapies. Given the superior tolerability of complementary and alternative therapy over conventional drug therapy, phytotherapeutic and homoeopathic remedies represent a potentially valuable treatment avenue in the management of sleep disorders, mild panic attacks, psychovegetative complaints, and depression. These alternative treatment methods can be used concomitantly with specific therapies addressing the underlying disease along with additional measures such as sleep hygiene, autogenic training, sports, identification of psychosocial burdens and appropriate psychotherapy (Kraft 2010).
The current observational study was undertaken to evaluate the safety and effectiveness of PASCONAL® NERVENTROPFEN on these nervous conditions in both adults and children.
METHODS
Study Design
This prospective, non-interventional, non-randomised, cohort study was conducted between 1st May and 31st August 2010 by 71 physicians. Male and female patients aged 1 to 89 years suffering from nervous disturbances and sleep disorders were treated with the homoeopathic combination product PASCONAL® NERVENTROPFEN (PASCOE pharmazeutische Praeparate GmbH, Giessen, Germany) in everyday practise. Treatment and dosage were decided by the therapist. No specific inclusion and exclusion criteria were defined due to the character of a non-interventional study. Data assessment included a physician-completed questionnaire on three occasions: at baseline (beginning of the observation period; visit one), approximately two weeks later (visit two) and approximately four weeks later (end of the observation period; visit three).
The study was registered with the Protocol Registration System ClinicalTrials.gov (NCT01125605). All participants and their parents gave written, informed consent.
Treatment
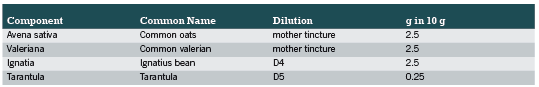
The patients were treated with the homoeopathic combination product PASCONAL® NERVENTROPFEN (ingredients listed in Table 1-Components of PASCONAL® NERVENTROPFEN and their dilutions). This product is indicated for the use in sleep disorders due to nervous restlessness according to its active ingredients (as listed in Felter´s Eclectic Materia Medica). Dose and duration of treatment were left to the respective physician’s discretion.
Outcome measures and Analyses
The data presented in this study are based on the data collected from the physician-completed questionnaires. The data were documented on at least two visits or on three visits at best. At the first visit, demographic data (sex, age, body height, body weight) were collected, as well as data on the duration of disease, previous therapies of the inclusion diagnosis, effectiveness and tolerability of previous therapies, relevant concomitant diseases/medication and the severity of symptoms. At visits two and three, dosage of the study medication and/or other medications, severity of 12 symptoms (nervousness/restlessness, irritability/eccentricity, sleep disorders, fitful sleep, hyperactivity, nocturnal activity, lack of concentration/forgetfulness, tiredness, discontent, listlessness, gastrointestinal problems, and headache/pressure), and effectiveness and tolerability of the study medication were recorded. If the study was completed on visit two, the effectiveness of PASCONAL® NERVENTROPFEN and adverse drug reactions were documented. Otherwise, these data were collected on visit three.
The severity of symptoms was graded on a four-point scale from 0 (no complaints) to 3 (strong complaints). The change from baseline to the end of the observation was calculated for the individual symptom scores and for the sum score of all 12 individual symptom scores (0 – 36).
Additionally, the improvement of the symptoms versus baseline was assessed at the end of the observation on a scale that ranged from improved, to no change, to deteriorated.
Statistics
Due to the character of an observational study, no hypotheses were specified and all conclusions were drawn from descriptive data analysis. The following values were computed, depending on the type of parameter: frequency data (absolute and relative frequencies), proportionally scaled measured values (median, 25% and 75% quartile, arithmetic mean, standard deviation, variance, minimum, maximum, number of valid data and number of missing data), and pre- / post-comparisons for data concerning symptoms.
Additionally, the main efficacy variables were analysed by explorative statistics. Parameters for analysis included the sum score of 12 clinical symptoms as well as the five individual symptoms nervousness/restlessness, irritability/eccentricity, sleep disorders, fitful sleep, and hyperactivity. Explorative statistical analysis was based on the change of the sum score between baseline (visit one) and the last documented visit (visit two or visit three), according to the LOCF (Last observation carried forward) principle. The change was calculated as “value pre minus value post”, which means that positive values indicate a decrease of the score (corresponding to an improvement). The sum score was also analysed with respect to age, gender, concomitant medication and treatment duration (< 4 weeks versus ≥ 4 weeks).
The one-sample t-test (two-sided) was used to test the null hypothesis that the changes from baseline were equal to zero. This analysis was performed for the total population and for each of the considered strata (age, gender, concomitant medication, treatment duration).
Analysis of covariance (ANCOVA) was applied to investigate the differences in the changes of the sum score between the various subgroups. The GLM (Generalized Linear Model) procedure of the SAS® system (with type III sums of squares) was used after including the baseline value of the sum score as a covariate in the underlying statistical model.
With respect to age, two types of analysis were carried out: 1) all four subgroups (1 to 6 years, 7 to 11 years, 12 to 17 years and ≥ 18 years); and 2) < 18 years and ≥ 18 years.
In order to show the results when baseline adjustment is omitted, the two-sample t-test (two-sided) was performed in addition to ANCOVA (only for pair-wise comparison).
RESULTS
A total of 325 patients were enrolled in the study and their treatment was documented during the observational study by 71 physicians. Baseline characteristics are summarised in Table 2. The inclusion diagnosis was made an average of two years ago and female patients suffered longer from nervousness than male patients (2.3 years versus 1.3 years). Two hundred forty five patients reported receiving previous therapies due to the inclusion diagnosis (11.1% only drug therapy; 24% only physical therapy/other therapy; 40.3% drug therapy and physical therapy/other therapy). The effectiveness of these previous therapies was mostly rated as “moderate” (58.4%) or “with no effect” (17.6%), while only 22.4% judged the effectiveness as “good” or “very good”. The tolerability of the previous therapies was rated as “very well” in 73.1% and only 4.5% stated poor tolerability. 70 patients continued their concomitant medication (92 medications in total) due to the inclusion diagnosis. It is shown that most of these continued drugs (43,5%) were all kinds of psychopharmaceuticals followed by all kinds of hypnotics/sedatives (40.2%)

The patients were treated with a mean daily dosage of three times a day 10 gtt PASCONAL® NERVENTROPFEN. The majority of patients were treated within the dosage recommendation as described in the SPC of PASCONAL® NERVENTROPFEN and the remaining patients were treated above the dosage recommendation.
In total, 12 different symptoms were observed during the time of the study, namely nervousness/restlessness, irritability/eccentricity, sleep disorders, hyperactivity, fitful sleep, nocturnal anxiety, lack of concentration/forgetfulness, tiredness, listlessness, discontent, gastrointestinal problems, and headache/head pressure. The change in the severity of each symptom between the start and end of the observation period is shown in Table 3. For the symptoms of nervousness/restlessness, irritability/eccentricity, sleep disorders, and fitful sleep, the majority of patients stated moderate or strong complaints (75.4%, 63.7%, 70.5% and 69.2%, respectively) at the start of the observation. These symptoms improved during the course of the study, with 87.7%, 88.9%, 84% and 87.4% of the patients, respectively, stating that they had no complaints anymore or only suffered from mild complaints.
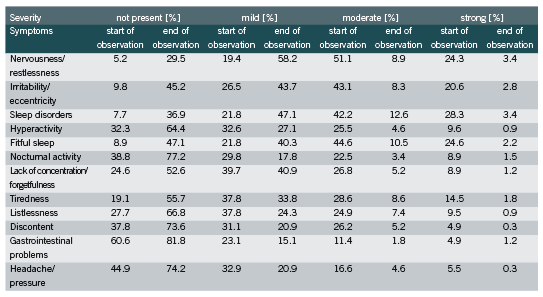
The other symptoms were less pronounced; for example, only 16.3% and 22.1% of the patients suffered from moderate and strong gastrointestinal problems and headache/pressure, respectively. Nevertheless, an improvement in at least 65% of the patients was observed for each symptom at the end of the observational study (Figure 1). In all symptoms less than 3% deterioration was seen.
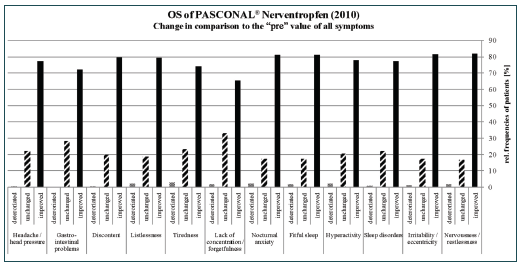
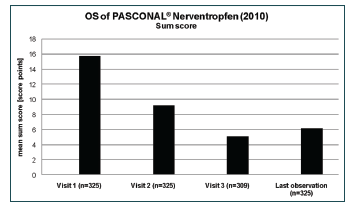
All symptoms were summarised to one “sum score”. The value decreased from and initial score of 15.8 to a score of 6.2 at the end of the study (Figure 2). The explorative analysis of the sum score was carried out with respect to age, gender, concomitant medication, and treatment duration. The difference between males and females in the mean decreases of the sum score (9.3 vs. 9.7) was not significant (p = 0.6115). The global test for differences between the four age groups did not show a significant result (p=0.0960). For a more robust analysis, all patients <18 years were combined into one age group. The mean baseline values of the sum score for the two resulting groups were 13.5 (<18 years) and 16.4 (≥18 years), respectively. Although the difference in the mean decreases was only 0.3 points, ANCOVA with baseline adjustment produced a significant result (p= 0.0238). The result of the analyses showed that the baseline value of the scan had a significant impact (p<0.0001) on the observed change.
Although the mean baseline values were comparable between patients who received concomitant medication due to the inclusion diagnosis and patients who did not (15.9 vs. 15.7), the decrease of the sum score was considerably more pronounced in the latter group (8.2 vs. 9.9). The difference between both subgroups of patients was significant (p = 0.0033).
Patients who received PASCONAL® NERVENTROPFEN for less than four weeks showed a considerably lower decrease of the sum score than patients who had been treated for four weeks or more (6.9 vs. 10.2). The difference between both subgroups of patients was significant (p = 0.0002).
The five individual symptoms, nervousness/restlessness, irritability/eccentricity, sleep disorders, fitful sleep, and hyperactivity, were analysed with respect to the direction of change between baseline and the last documented visit by means of the categories “improved”, “unchanged” and “worsened”. Differences between subgroups were analysed with Fisher’s exact test (two-sided) for stratification by age (<18 years vs. ≥ 18 years), concomitant medication (due to inclusion diagnosis), and duration of treatment with PASCONAL® NERVENTROPFEN. The age of the patients had no significant effect on the five selected symptoms. In patients who did not receive concomitant medication due to the inclusion diagnosis, a comparatively more positive and significant (p < 0.05) effect on the symptoms nervousness/restlessness and irritability/eccentricity was observed. The corresponding p-values for the remaining three symptoms were > 0.05. The effect of PASCONAL® NERVENTROPFEN on all five individual symptoms was more pronounced in patients who were treated for four weeks or more than in patients with a shorter treatment period. All p-values for comparison between the two subgroups were < 0.05.
Overall, PASCONAL® NERVENTROPFEN was well tolerated by 96.0% of the patients in the first observation interval and by 92.9% in the second observation interval. Fifteen patients reported moderate or poor tolerability of PASCONAL® NERVENTROPFEN. Seven adverse events were reported with possible relationship to study treatment (2x nausea, 2x headache, 2x tiredness and 1x tachycardia/acid reflux ), four with probable relationship (2x gastrointestinal disorders, 1 x severe tiredness and 1 x heartburn), two events with relationship unlikely (1 x aggressivity, 1 x stomach ache,) and only one with a certain relationship to the treatment (tongue and mucosa under tongue affected by alcohol concentration of the drops). In one case the relationship was not assessable. None of the adverse events required additional medical treatment.
DISCUSSION
The results of this observational study reveal that the homoeopathic combination preparation, PASCONAL® NERVENTROPFEN, is effective in the symptomatic treatment of sleep disorders due to nervous restlessness.
The baseline data revealed that the majority of patients suffered from nervousness/restlessness, irritability/eccentricity, sleep disorders, and fitful sleep. The majority of patients had already received therapies due to the inclusion diagnosis and half of those therapies were pharmaceuticals. Astonishingly, over 75% of the patients who used previous therapies reported either no effect or moderate effectiveness of these therapies.
After treatment with the homoeopathic combination preparation, a clear reduction in the symptom severity was observed for all analysed symptoms. Most patients experienced more than a 50% reduction in moderate and strong symptoms. Upon analysis of the sum score, it can be concluded that from start until the end of the observation, a reduction by 9.6 points could be observed, which corresponds to a decrease by 60%.
The explorative analysis showed a connection between the sum score and age groups, concomitant medication and therapy duration, respectively. Patients over 18 years of age had a significant reduction of the mean sum score compared with the group of patients less than 18 years. However, it is important to point out that both age groups differ in the number of patients (72 under 18 years versus 253 patients over 18 years).
Patients who did not take any concomitant medication due to the inclusion diagnosis showed a higher decrease in the mean value of the sum score at the end of the observation. Compared with the group of patients who received concomitant medication, this result was significant.
Therapy duration influenced the sum score value: patients who had been treated for four weeks and more showed a considerably higher decrease of the sum score than patients who received the homeopathic combination preparation less for than four weeks. The difference between both subgroups of patients was significant.
Age had no influence on the direction of change (improved, unchanged or worsened) between baseline (visit 1) and the last documented visit of the five symptoms (nervousness/restlessness, irritability/eccentricity, sleep disorders, fitful sleep and hyperactivity). However, patients who did not receive concomitant medication showed a significant effect on the direction of change in nervousness/restlessness and irritability/eccentricity. For the remaining three symptoms, no significant effect could be observed. Interestingly, an effect on all five symptoms could be shown in patients who were treated four weeks or more.
The therapy was well tolerated, which was to be expected based on the experience gained in pharmacovigilance surveillance of the product.
LIMITATIONS
Observational studies have several limitations (Black 1996), including the absence of a placebo group. This can create bias and can also mask cause and effect relationships or suggest correlations where there are none. Therefore, no definite conclusions can be extrapolated from these studies.
AUTHORS’ CONTRIBUTIONS
Financial support for the analysis was provided by PASCOE pharmazeutische Präparate GmbH, Germany. The sponsor had influence on the conduct of the analysis in so far as the management and evaluation of the data was conducted in the department of Clinical Research of PASCOE pharmazeutische Präparate GmbH. The explorative statistics were done by D. Schremmer, GKM Gesellschaft für Therapieforschung mbH, Germany.
LITERATURE
Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data 2004; (343): 1-19.
Berger H, Fritz U. Medication in acute psychiatric treatment. Psychiatr Prax. 2004; 31 (2): 68-73.
Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996; 312(7040): 1215-8.
Coholic D. The Helpfulness of Spiritually Influenced Group Work in Developing Self-Awareness and Self-Esteem: A Preliminary Investigation.” The Scientific World Journal. 2005; 5: 789–802.
Hankey A. CAM and Post-Traumatic Stress Disorder. eCAM. 2006
Kraft K. Schlaf- und psychische Störungen im Kindesalter. Z Phytotherapie. 2007; 28: 235-7.
Kraft K. Depressionen und depressive Verstimmungszustände. MMW Fortschr Med. 2010;152(8): 20-21.
Kumar S, Anupam S. Anti-anxiety Activity Studies on Homoeopathic Formulations of Turnera phrodisiaca Ward. eCAM. 2005; 2 2(1): 117-119.
Meerschaut Lvd, Andrea S. The Homeopathic Preparation Nervoheel N can Offer an Alternative to Lorazepam Therapy for Mild Nervous Disorders. eCAM. 2007
Rosenberg RP. Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies. Ann. Clin. Psychiatry. 2006; 18: 49-56.
Schneider B, Hanisch J, Weiser M. Complementary medicine prescription patterns in Germany. Ann Pharmacother. 2004; 38: 502–507.
Tan KR, Brown M, Labouebe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Luscher C. Neural bases for addictive properties of benzodiazepines. Nature. 2010; 463: 769-774.
Ventegodt S, Clausen B, Merrick J. Clinical Holistic Medicine: The Case Story of Anna. I. Long-Term Effect of Childhood Sexual Abuse and Incest with a Treatment Approach. The Scientific World Journal. 2006; 6: 1965-1976.
Waldschutz R, Klein P. The homeopathic preparation Neurexan vs. valerian for the treatment of insomnia: an observational study. Scientific World Journal. 2008; 8: 411-20.









