Diet in inflammatory bowel disease prevention and management A review
Abstract
As a result of inconsistent health improvement and severe adverse effects associated with the use of current drug therapies, inflammatory bowel disease (IBD) patients have turned to alternative treatments such as diet modification to help alleviate IBD disease symptoms. This review discusses the role of diet in preventing and managing IBD symptoms. Specifically, the influences of dietary intake of gluten, fiber, Fermentable Olio-, Di-, and Mono-saccharides and Polyols (FODMAPs), dairy, meat, and polyunsaturated fats are elucidated. This review concludes that though substantial evidence exists for each of these topics and their role in IBD, more research should be conducted in order to further delineate the role of diet in IBD symptom development and control. Recommendations include careful monitoring and coordination of diet on a case-per-case basis in patients with IBD in order to decrease adverse IBD-associated symptoms. This can be achieved through elimination and reintroduction diets, which may allow practitioners to identify dietary sources of IBD flare-ups.
Introduction
Inflammatory bowel disease (IBD) is a chronic disorder characterized by inflammation within the gastrointestinal (GI) tract as a result of dysregulated interactions between the mucosal immune system and intestinal microorganisms in genetically predisposed individuals (Hyun 2006, Neuman 2007). IBD manifests itself as either ulcerative colitis (UC), with localized inflammation and ulcerative lesions occurring in superficial layers of the colon, or Crohn’s disease (CD), with inflammation and lesions occurring transmurally and sporadically along the entire length of the GI tract (Hyun 2006, Neuman 2007). Chronic inflammation leads to clinical symptoms such as weight loss, abdominal pain, and bloody diarrhea, all of which can negatively impact patients’ quality of life (Hendrickson 2002).
IBD can affect individuals of all ages, but is most commonly diagnosed in the second or third decade of life. In 2012, the prevalence of individuals with IBD was 233,000 in Canada, with an approximate incidence rate of 10,200 cases per year. The economic cost of IBD in Canada has been estimated at $2.8 billion per year, considering both the direct and indirect costs of healthcare (Rocchi 2012).
There is currently no cure for IBD (Mulder 2013). Instead, treatment approaches focus on symptom management, with the predominant therapy being 5-aminosalicyclic acid, a mild anti-inflammatory, in combination with steroids (Panaccione 2008). Following the failure of this therapy option, stronger immunosuppressive therapies such as thiopurine analogues, methotrexate, and calcineurin inhibitors are prescribed in order to ameliorate disease symptoms (Panaccione 2008). However, data on these immunosuppressive therapies has either been inconclusive or has demonstrated the drugs’ lack of ability to induce and maintain remission (Khan 2011). Although there is evidence that one thiopurine analogue, azathioprine, is moderately effective at preventing relapse, this effect disappears after four years of use (Bouhnik 1996, Khan 2011). The numerous side effects of these drugs prevent this class of treatment from being an ideal option for long-term management (de Boer 2007, Haslam 2000, Loftus 2004, Mason 2013). For example, severe adverse effects such as hypertension, lethargy, and lower respiratory tract infection have been found in 51% of participants taking the immunosuppressant cyclosporin for long term treatment of IBD (Haslam 2000).
As a result of inconsistent health improvement and the aforementioned severe adverse effects of current drug therapies, IBD patients have turned to alternative treatments, such as diet modification, to help alleviate disease symptoms (Hendrickson 2002). These altered diets can include or exclude many items, such as gluten, fiber, FODMAP carbohydrates, dairy, meat, and polyunsaturated fats. Though no one diet has been proven to help treat all cases of IBD, the focus of this literature review will be to collect and summarize current research on diet-based approaches to IBD prevention and treatment.
Gluten
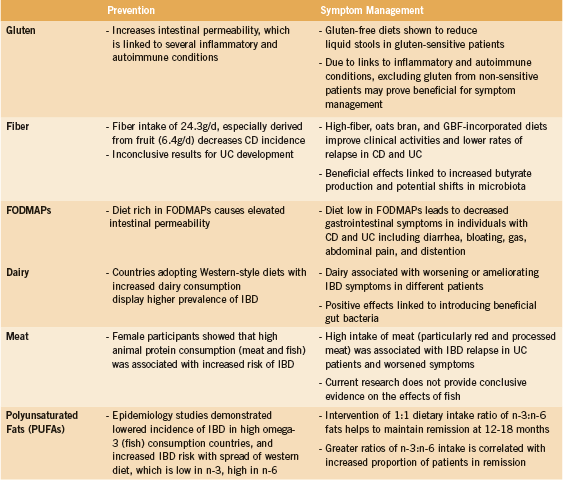
Though celiac disease is not an IBD, studies have shown an increased incidence of non-celiac gluten sensitivity (NCGS) (Watanabe 2013) and celiac disease (Oxford 2013, Tavakkoli 2012) in IBD patients compared to non-IBD patients (13% vs. 1% and 9% vs. 1%, respectively). NCGS and celiac disease often present with similar symptoms, which can be ameliorated by the adoption of a gluten-free diet; however, the two are differentiated by distinct clinical features (Kabbani 2014). Celiac disease is often characterized by the presence of three elements: 1) a genetic predisposition to the disease, determined by the expression of HLA-DQ2 or HLA-DQ8 proteins, 2) gluten as the precipitating factor to symptoms, and 3) autoantibodies against tissue transglutaminase (tTG) (Schuppan 2013). The presence of tTG leads to structural changes to a-gliadin, a protein component of gluten, which is then presented on HLA-DQ2 or HLA-DQ8 to cytotoxic CD8+ T cells in the lamina propria of the gut (Schuppan 2013). Additionally, helper CD4+ T cells in the lamina propria release pro-inflammatory cytokines, which leads to intestinal inflammation, similar to that observed in IBD (Schuppan 2013).
Gluten-containing foods have been linked to worsened symptoms in IBD patients (Asakura 2008, Brown 2010, Mishkin 1997, Riordan 1993, Triggs 2010). In cases of IBD patients with NCGS, one study found that eliminating gluten from the diet significantly reduced liquid stools per day (from 6.2 to 1.5) compared to gluten-eating controls (from 4.0 to 4.7) (Watanabe 2013). Though this evidence pertains to NCGS IBD patients, research has shown that gluten consumption is linked to increased intestinal permeability and inflammation. Due to the association between intestinal permeability and diseases such as rheumatoid arthritis, multiple sclerosis, and depression (de Punder 2013), it may be beneficial for non-gluten-sensitive IBD patients to also exclude gluten from their diets to reduce the risk of developing the aforementioned comorbidities.
Dietary Fiber
The efficacy of fiber intake as a dietary intervention for IBD has been of interest due to prospective studies showing decreased CD risk in patients with high vs. low (24.3g/d, 11.6g/d) fibre consumption (Ashwin 2013). One study following 170,000 women over 26 years found a 40% CD risk decrease in those with high fibre intake, especially fiber derived from fruits (6.4g/d) (Ashwin 2013). These results are supported by additional studies (Hou 2011). Although dietary fiber has been associated with decreased UC incidence in some studies, few trials have yielded statistically significant results (Ashwin 2013, Hou 2011).
A retrospective study conducted on patients with CD demonstrated that individuals treated with a fiber-rich (32.6g/d), unrefined-carbohydrate diet and conventional medication experienced a reduction in hospital visits (total 111 vs. 533) and surgeries required (1 vs. 5) when compared to patients in a drug-only treatment group (Heaton 1979). More recently, a study administering 20g of fiber per day through oat bran in bread to quiescent UC patients displayed improvement in abdominal pain and gastroesophageal reflux with no adverse effects or relapse activity observed (Hallert 2003). Open label studies where patients with mild to moderate UC consumed 20g/d of germinated barley foodstuff (GBF) in conjunction with standard drug treatments resulted in various clinical activity improvements including decreased diarrhea, less blood in stool, and lower cumulative recurrence rates (Bamba 2002, Hanai 2004).
Though the mechanisms through which these foods benefit IBD is not fully elucidated, possible explanations suggest that fiber can indirectly affect IBD through interacting with the intestinal microbiome and increasing production of butyrate (Flint 2008). Fiber intake results in residential bacteria fermentation, which increase short chain fatty acid (SCFA) production in the colon (Viladomiu 2013). SCFAs, such as butyrate, down-regulate and modulate intestinal inflammatory factors, cytokines interleukin-6 (IL-6), IL-8, and tumor necrosis factor-α (TNF-α), all of which play a role in intensifying inflammation and aggravating intestinal tissue; SCFAs therefore indirectly prevent damage to colonic mucosa (Galvez 2005, Rodríguez-Cabezas 2002, Viladomiu 2013). Dietary fiber intake is also associated with promoting anti-inflammatory actions, as it increases the percentage of Treg immune cells in the gut and decreases interferon-y (IFNy) production (Galvez 2005, Viladomiu 2013). The therapeutic effects of dietary fiber may also be due to shifting microbiota profiles through favouring the survival of bacteria capable of SCFA fermentation. This is suggested by research reporting that vegans and vegetarians, who may consume more fibre through fruits and vegetables, have lowered bifidobacterium compared to omnivores (Zimmer 2012). In contrast, ulcerative colitis patients have increased bifidobacterium, as well as increased bacteriodes, enterococcus, and clostridium species in the gut compared to controls (Wang 2014, Zhang 2013).
FODMAPs
A diet with decreased amounts of highly fermentable, but poorly absorbed short chain carbohydrates and polyols consumed, known as the FODMAPs diet (Fermentable Olio-, Di-, and Mono-saccharides and Polyols) is a relatively new method of treating IBD (Gibson 2005). FODMAPs include fructooligosaccharides (wheat, legumes), lactose (milk), fructose (fruits, honey), galactans (legumes) and sorbitol (artificial sweetener) (Gearry 2009, Gibson 2005). It has been found that a diet rich in FODMAPs causes elevated intestinal permeability, which is a biomarker of susceptibility for CD (Gibson 2005). FODMAPs in the small intestine are poorly absorbed, and exert an osmotic effect by drawing water into the large intestine due to their small molecular size (Barrett 2010, Gearry 2009). The FODMAPs are then fermented by the colonic microflora in the large intestine, producing hydrogen and/or methane gas (Barrett 2010, Gearry 2009). The increase in fluid and gas in the intestines results in diarrhea, bloating, gas, abdominal pain, and distention (Barrett 2010). A diet low in FODMAPs can lead to a decrease in these gastrointestinal symptoms in individuals with CD and UC (Gearry 2009). However, this diet has been shown to have no or a negative effect on constipation in study subjects (Gearry 2009, Gibson 2005). This diet requires time and dedication to achieve desirable outcomes, and is more favorable for individuals with higher education and who have more time to spend on food shopping (Gearry 2009).
In addition to FODMAPs, an older diet popularized by Elaine Gottschall’s book Breaking the Vicious Cycle: Intestinal Health Through Diet, called the Specific Carbohydrate Diet (SCD), has been used in the treatment of IBD. Like FODMAPs, the SCD limits the use of certain complex carbohydrates such as disaccharides and polysaccharides, on the basis that these may not be completely digested by the body, and may serve as fuel for overgrowth of bacteria and yeast instead. Although most evidence is anecdotal, and there is limited scientific evaluation of the SCD, a recent study assessed the effectiveness of the SCD in children with Crohn’s disease (Suskind 2014). A retrospective chart analysis, this study included seven children aged seven to 16 years of age with Crohn’s disease receiving the SCD for an average of 14.6 months, and no immunosuppressive medications. Disease severity ranged from mild to severe. The authors stated that “Although the exact time of symptom resolution could not be determined through chart review, all symptoms were notably resolved at a routine clinic visit three months after initiating the diet. Each patient’s laboratory indices, including serum albumin, C-reactive protein, hematocrit, and stool calprotectin, either normalized or significantly, improved during follow-up clinic visits” (Suskind 2014).
Dairy
In countries adopting “Western-style” diets, which include more high-fat dairy consumption, the prevalence of IBD has also increased (Asakura 2008). This suggests that increased dairy consumption can lead to greater IBD incidence, though this relationship is not observed in all countries (Jantchou 2010). As with other self-reported food intolerances in IBD, dairy has not been shown to have consistent effects in all IBD patients. Some studies (Brown 2010, Cohen 2013, Mishkin 1997, Riordan 1993, Triggs 2010, Wright 1965) have reported that dairy milk is associated with worsened diarrhea, bloating, and gas in many patients. Conversely, dairy yoghurt has been seen to ameliorate these symptoms in many patients (Cohen 2013, Triggs 2010), highlighting the possibility that the positive effects of introducing beneficial gut bacteria surpass the negative effects of dairy. Dairy should be avoided with caution, as it has been shown that patients who perceive dairy to be harmful for them do not meet their recommended daily allowance (RDA) for calcium intake as frequently as dairy-consumers (87.6% vs. 105.8% RDA) (Vernia 2013).
Meat
Animal protein has been shown to have an effect on both IBD incidence and IBD health outcomes (Hou 2011, Jantchou 2010, Jowett 2004, Mishkin 1997, Triggs 2010). One prospective study comprised of 67,581 female participants showed that high animal protein consumption including meat and fish was associated with a significantly increased risk of being diagnosed with IBD (HR 3.31 and 3.03 for total and animal protein, respectively) (Jantchou 2010). Regarding clinical activity, a study conducted on UC patients demonstrated that high intake of meat (OR compared with low intake 3.74), particularly red and processed meat (OR 6.88), was associated with IBD relapse (Jowett 2004). Additionally, many studies point to a link between red meat and worsened symptoms (Cohen 2013, Hou 2011, Jantchou 2010, Jowett 2004, Triggs 2010). Current research does not provide conclusive evidence on the effects of fish. Some note that fish is beneficial to many patients (Hou 2011, Triggs 2010) while others claim it is not (Hou 2011, Mishkin 1997).
Polyunsaturated Fats
Epidemiological evidence has illustrated a link between dietary fat intake and the incidence of IBD. Studies have shown a lowered incidence of IBD in the Eskimo population, whose diet is rich in omega-3 n-3 polyunsaturated fats (PUFA) (Kromann 1980), and an increased risk of IBD in Western populations with a diet characterized by high n-6 PUFA and a decreased ratio of n-3:n-6 fat consumption (Hou 2011, Lupien 1994, Simopoulos 1994). In addition, higher intake of linoleic acid (n-6 PUFA) is associated with increased UC risk, while oleic (n-9 MonoUFA) and docosahexaenoic acids (DHA n-3 PUFA) have been associated with a decreased UC risk (Tjonneland 2009).
A 2010 study by Uchiyama et al. investigated the impact of dietary omega-3 and -6 intake ratios on maintaining remission in individuals with IBD. These fats were of particular interest as N-6 PUFAs are considered to be pro-inflammatory given that linoleic acid, the major n-6 PUFA, increases inflammatory mediators, including prostaglandin E2 and leukotriene B4 (Rachmilewitz 1982, Sharon 1984). In addition, animal studies have shown that n-3 PUFAs, which include a-linolenic acid, DHA, and eicosapentaenoic acid, are generally antiinflammatory (Hassan 2010, Ibrahim 2011). The study used 230 individuals with UC and CD who had achieved remission from drug therapy and prescribed them a dietary regimen that provided a n-3:n-6 fat intake ratio of 1:1. All steroids and immunosuppressants were gradually removed after achievement of remission, except for 5-ASA and thiopurines. The results showed that the dietary regimen resulted in changes of n-3:n-6 ratios in the patients’ erythrocyte membranes. Consequently, remission rates after 12-18 months were 79.7% and 54.3% in patients who maintained a n-3:n-6 membrane ratio of greater than 0.65, and less than 0.65, respectively (Uchiyama 2010).
Discussion
This review highlights the implications gluten, fiber, FODMAP carbohydrates, dairy, meat, and polyunsaturated fats have on IBD (Appendix I). Effective dietary strategies for IBD seem to decrease existing GI inflammation (high fiber, GBF and n-3 PUFA, or low FODMAPs), or decrease food-induced immune activation (low gluten, dairy, or meat).
Conclusions for clinical consideration are difficult to draw due to various limitations present in the research. For instance, though studies on dietary fibre indicate fruits as a preferred source of fibre in controlling IBD symptoms, the FODMAPs diet calls for the exclusion of certain fruits, given their potential for adverse IBD-related outcomes. The emerging evidence in these two areas suggest that the therapeutic effect of high-fiber diets may be limited by the inclusion of high FODMAP containing foods. Thus, further studies on the effect of high-fiber, low- FODMAP diets are needed. Another limitation of dietary studies lies in the inherent difficulty of adherence to dietary interventions; patients may introduce bias into observed results if diet regimens are not properly followed.
Although there are challenges to the use of dietary interventions to treat IBD, there is compelling evidence for the use of these treatments in practice. The use of diet alone has been shown to reduce the incidence of flare-ups (Wild 2007), increase remission time (7.5 vs. 3.8 months) and decrease relapse rates at 2 years (62% vs. 79%) when compared to 40 mg doses of prednisone daily (Riordan 1993). Administration of diet in conjunction with standard anti-inflammatory treatment can also lead to greater symptom alleviation.
Ultimately, dietary modulation in the prevention and control of IBD should be tailored to individual patients. This may be accomplished through the use of elimination and reintroduction diets, which allow practitioners to identify the food sources causing IBD flare-ups (Candy 1995). This review provides a starting point for such interventions.
Conclusion
This review has discussed the role of diet in managing IBD symptoms, specifically, the influences of gluten, fiber, FODMAP carbohydrates, dairy, meat, and polyunsaturated fat consumption. Though much evidence exists for each of the topics discussed herein, more research should be conducted in order to further delineate the role of diet in IBD symptom development and control. Given the studies explored in this review, it is evident that ingestion of these foods should be carefully monitored and coordinated in patients with IBD in order to decrease and modulate adverse IBD-associated symptoms.
References
Asakura H, Suzuki K, Kitahora T, Morizane T. Is there a link between food and intestinal microbes and the occurrence of Crohn’s disease and ulcerative colitis? Journal of Gastroenterology and Hepatology. 2008;23(12):1794-1801.
Bamba T, Kanauchi O, Andoh A, Fujiyama Y. A new prebiotic from germinated barley for nutraceutical treatment for ulcerative colitis. J Gastroenterol Hepatol. 2002;17(8):818-24.
Barrett JS, Gearry RB, Muir JG, Irving PM, Rose R, Rosella O, et al. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Alimentary Pharmacology and Therapeutics. 2010;31: 874–82.
Bouhnik Y, Lémaan M, Mary JY, Scemama G, Taï R, Matuchansky C, et al. Long-term follow-up of patients with Crohn’s disease treated with azathioprine or 6-mercaptopurine. Lancet. 1996;347(8996):215-9.
Brown AC, Roy M. Does evidence exist to include dietary therapy in the treatment of Crohn’s disease? Expert review of Gastroenterology and Hepatology. 2010;4(2):191-215.
Candy S, Borok G, Wright JP, Boniface V, Goodman R. The value of an elimination diet in the management of patients with ulcerative colitis. South African Medical Journal. 1995;85(11):1176-9.
Cohen AB, Lee D, Long MD, Kappelman MD, Martin CF, Sanders RS, et al. Dietary Patterns and Self-Reported Associations of Diet with Symptoms of Inflammatory Bowel Disease. Digestive Diseases and Sciences. 2013;58(5):1322-8.
de Boer NK, van Bodegraven AA, Jharap B, de Graaf P, Mulder CJ. Drug insight: pharmacology and toxicity of thiopurine therapy in patients with IBD. Nature Clinical Practice Gastroenterology and Hepatology. 2007;4(12):686-94.
de Punder K, Pruimboom L. The Dietary Intake of Wheat and other Cereal Grains and Their Role in Inflammation. Nutrients. 2013;5(3):771-87.
Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nature Reviews. Microbiology. 2008;6(2):121-31.
Galvez J, Rodríguez-Cabezas ME, Zarzuelo A. Effects of dietary fiber on inflammatory bowel disease. Molecular Nutrition and Food Research. 2005;49(6):601-8.
Gearry RB, Irving PM, Barrett JS, Nathan DM, Shepherd SJ, Gibson PR. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease—a pilot study. Journal of Crohn’s and Colitis. 2009;3(1):8-14.
Gibson PR, Shepherd SJ. Personal view: food for thought – western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Alimentary Pharmacology and Therapeutics. 2005;21(12):1399-1409.
Hanai H, Iiad T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clinical Gastroenterology and Hepatology. 2006;4(12):1502-6.
Hanai H, Kanauchi O, Mitsuyama K, Andoh A, Takeuchi K, Takayuki I. Germinated barley foodstuff prolongs remission in patients with ulcerative colitis. Int J Mol Med. 2004;13(5):643-7.
Hallert C, Bjorck I, Nyman M, Pousette A, Granno C, Svensson H. Increasing fecal butyrate in ulcerative colitis patients by diet: controlled pilot study. Inflamm Bowel Dis. 2003;9(2):116-21.
Hassan A, Ibrahim A, Mbodji K, Coëffier M, Ziegler F, Bounoure F, et al. An α-linolenic acid-rich formula reduces oxidative stress and inflammation by regulating NF-κB in rats with TNBS-induced colitis. Journal of Nutrition. 2010;140(10):1714-21.
Haslam N, Hearing SD, Probert CS. Audit of cyclosporin use in inflammatory bowel disease: limited benefits, numerous side-effects. European Journal of Gastroenterology and Hepatology. 2000;12:657–60.
Heaton KW, Thornton JR, Emmett PM. Treatment of Crohn’s disease with an unrefined-carbohydrate, fibre-rich diet. Br Med J. 1979;2(6193):764-6.
Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clinical Microbiology Reviews. 2002;15(1):79-94.
Hou JK, Abraham B, El-Serag H. Dietary Intake and Risk of Developing Inflammatory Bowel Disease: A Systematic Review of the Literature. The American Journal of Gastroenterology. 2011;106:563-73.
Hyun JG, Mayer L. Mechanisms underlying inflammatory bowel disease. Drug Discovery Today: Disease Mechanisms. 2006;3(4):457-62.
Ibrahim A, Mbodji K, Hassan A, Aziz M, Boukhettala N, Coeffier M, et al. Anti-inflammatory and anti-angiogenic effect of long chain n-3 polyunsaturated fatty acids in intestinal microvascular endothelium. Clinical Nutrition. 2011;30(5):678-87.
Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault M, Carbonnel F. Animal Protein Intake and Risk of Inflammatory Bowel Disease: The E3N Prospective Study. The American Journal of Gastroenterology. 2010;105:2195-2201.
Jowett SL, Seal CJ, Pearce MS, Phillips E, Gregory W, Barton JR, et al. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut. 2004;53(10):1479-84.
Kabbani TA, Vanga RR, Leffler DA, Villafuerte-Galvex J, Pallav K, Hansen J, et al. Celiac Disease or Non-Celiac Gluten Sensitivity? An Approach to Clinical Differential Diagnosis. The American Journal of Gastroenterology. 2014. doi: 10.1038/ajg.2014.41
Khan KJ, Dubinski MC, Ford AC, Ullman TA, Talley NJ, Moayyedi P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. The American Journal of Gastroenterology. 2011;106(4):630-42.
Kromann N, Green A. Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950-1974. Acta Medica Scandanavica. 1980;208(5):401-6.
Loftus CG, Egan LJ, Sandborn WJ. Cyclosporine, tacrolimus, and mycophenolate mofetil in the treatment of inflammatory bowel disease. Gastroenterology Clinics of North America. 2004;33(2):141-69.
Lupien JR, Richmond A, Randell M, Cotier JP, Ghazali A, Dawson R. Food, Nutrition and Agriculture: Edible Fats and Oils. Agriculture and Consumer Protection Department, Food and Agriculture Organization of the United Nations, 1994.
Mason M, Siegel CA. Do inflammatory bowel disease therapies cause cancer? Inflammatory Bowel Diseases. 2013;19(6):1306-21.
Mishkin S. Dairy sensitivity, lactose malabsorption, and elimination diets in inflammatory bowel disease. The American Journal of Clinical Nutrition. 1997;65(2):564-567.
Mulder DJ, Noble AJ, Justinich CJ, Duffin JM. A tale of two diseases: the history of inflammatory bowel disease. Journal of Crohn’s and Colitis. 2013. doi: 10.1016/j.crohns.2013.09.009
Neuman MG. Immune dysfunction in inflammatory bowel disease. Translational Research. 2007;149(4):173-86.
Oxford EC, Nguyen DD, Sauk J. Impact of Coexistent Celiac Disease on Phenotype and Natural History of Inflammatory Bowel Diseases. The American Journal of Gastroenterology, 2013;108(7):1123-9.
Panaccione R, Rutgeerts P, Sandborn WJ, Feagan B, Schreiber S, Ghosh S. Review article: treatment algorithms to maximize remission and minimize corticosteroid dependence in patients with inflammatory bowel disease. Alimentary Pharmacology and Therapeutics. 2008;28(6):674-88.
Rachmilewitz D, Ligumsky M, Haimovitz A, Treves AJ. Prostanoid synthesis by cultured peripheral blood mononuclear cells in inflammatory diseases of the bowel. Gastroenterology. 1982;82(4):673-9.
Riordan AM, Hunter JO, Cowan RE, Crampton JR, Davidson AR, Dickinson RJ, et al. Treatment of active Crohn’s disease by exclusion diet: East Anglian multicentre controlled trial. Lancet. 1993;342(8880):1131-4.
Rocchi A, Benchimol EI, Bernstein CN, Bitton A, Feagan B, Panaccione R, et al. Inflammatory bowel disease: a Canadian burden of illness review. Canadian Journal of Gastroenterology. 2012;26(11):811-7.
Rodríguez-Cabezas ME, Gálvez J, Lorente MD, Concha A, Camuesco D, Azzouz S, et al. Dietary fiber down-regulates colonic tumor necrosis factor alpha and nitric oxide production in trinitrobenzenesulfonic acid-induced colitic rats. The Journal of Nutrition. 2002;132(11):3263-71.
Schuppan D, Zimmer KP. The Diagnosis and Treatment of Celiac Disease. Deutsches Arzteblatt International. 2013;110(49):835-46.
Sharon P, Stenson WF. Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology. 1984;86(3):453-60.
Simopoulos AP. Is insulin resistance influenced by dietary linoleic acid and trans fatty acids? Free Radical Biology and Medicine. 1994;17(4):367–372.
Suskind DL, Wahbeh G, Gregory N, Vendettuoli H, Christie D. Nutritional therapy in pediatric Crohn disease: the specific carbohydrate diet. J Pediatr Gastroenterol Nutr. 2014 Jan;58(1):87-91.
Tavakkoli H, Haghdani S, Adilipour H. Serologic celiac disease in patients with inflammatory bowel disease. Journal of Research in Medical Sciences. 2012;17(2):154-8.
Tjonneland A, Overvad K, Bergmann MM, Nagel G, Linseisen J, et al. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case-control study within a European prospective cohort stu Gut. 2009;58(12):1606-11.
Triggs CM, Munday K, Hu R, Fraser AG, Gearry RB, Barclay ML, et al. Dietary factors in chronic inflammation: Food tolerances and intolerances of a New Zealand Caucasian Crohn’s disease population. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2010;690(1-2):123-38.
Uchiyama K, Nakamura M, Odahara S, Koido S, Katahira K, Shiraishi H, et al. N-3 polyunsaturated fatty acid diet therapy for patients with inflammatory bowel disease. Inflammatory Bowel Disease. 2010;16(10):1696-707.
Vernia P, Loizos P, Di Giuseppantonio I, Amore B, Chiappini A. Dietary calcium intake in patients with inflammatory bowel disease. Journal of Crohn’s and Colitis. 2013. doi: 10.1016/j.crohns.2013.09.008.
Viladomiu M, Hontecillas R, Yuan L, Lu P, Bassaganya-Riera J. Nutritional protective mechanisms against gut inflammation. The Journal of Nutritional Biochemistry. 2013;24(6):929-39.
Wang W, Chen L, Zhou R, Wang X, Song L, Huang S. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J Clin Microbiol. 2014;52(2):398-406.
Watanabe C, Komoto S, Hokari R, Kurihara C, Okada Y, Hozumi H, et al. Prevalence of serum celiac antibody in patients with IBD in Japan. Journal of Gastroenterology. 2013.
Wild GE, Drozdowski L, Tartaglia C, Clandinin MT, Thomson ABR. Nutritional modulation of the inflammatory response in inflammatory bowel disease – from the molecular to the integrative to the clinical. World Journal of Gastroenterology. 2007;13(1):1-7.
Wright R, Truelove SC. A controlled therapeutic trial of various diets in Ulcerative Colitis. British Medical Journal. 1965;2(5454):138–41.
Zhang T, Chen Y, Wang Z, Zhou Y, Zhang S, Wang P, Xie S, Jiang B. [Changes of fecal flora and its correlation with inflammatory indicators in patients with inflammatory bowel disease]. Nan Fang Yi Ke Da Xue Xue Bao. 2013 Oct;33(10):1474-7.
Zimmer J, Lange B, Frick JS, Sauer H, Zimmermann K, Schwiertz A. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur J Clin Nutr. 2012;66(1):53-60.