Diabetes Medications
Reduced risk of disease-related complications?
Abstract
It is not debatable that the elevated blood glucose levels associated with diabetes represent a serious risk marker for macrovascular and microvascular adverse developments and events, and that the risk increases with the average of the daily fluctuations. This provides the principal rationale for maintaining both fasting blood glucose and the indicator of the long- term average, glycated hemoglobin, at levels close to normal. More than a dozen prescription drugs are currently used individually or in combination to treat type 2 diabetes. They act by decreasing liver glucose production, increasing insulin secretion, increasing insulin sensitivity, inhibiting glucagon release and slowing the absorption of carbohydrates. These drugs decrease serum glucose and glycated hemoglobin with the goal of glucose reduction to targets approaching normal, a result that is generally believed will significantly decrease the incidence of the complication associated with the disease. Failure to achieve blood glucose control generally results in additional drugs and even insulin, and is frequently termed intensive treatment. As will be discussed, there are not only new studies but recent meta-analyses that have examined the impact of intensive blood glucose lowering on complications in type 2 diabetes. Evidence will be presented that in fact glucose lowering, either intensive or not, is a failed therapy if judged by the impact on clinical complications, a failure termed by some medical scientists the Diabetes Paradox.
Introduction
Even before the beginning of the 20th century, carbohydrate restriction was the established medical response to diabetes and obesity. This changed dramatically in the early 1960s when the hypothesis that fat and in particular saturated fat increased the risk of both heart disease and cancer was accepted as true without adequate evidence and profoundly altered diets. The macronutrients that were most effective in driving up blood sugar, elevating insulin, and viewed as causing insulin resistance, beta cell dysfunction and diabetes became the main components of the so-called healthy, low fat diet in the developed world. These were mostly carbohydrates and as the trend accelerated, these were more refined and had higher glycemic activity. Then drugs became available which would lower blood sugar and its daily average and offered a logical solution. The number of anti-hyperglycaemic agents increased rapidly. If used intensively, the drugs were able to bring glucose levels down to somewhat above normal and this was viewed as a success. Thus the blood glucose elevations associated with diets rich in carbohydrates were counteracted, once diabetes had developed, by daily dosing with antihyperglycemic drugs. The impact on complications, the principal reason for glucose control, is however a developing story and the subject of this article. In what follows, the evidence will be presented that once one has diabetes, controlling both the daily fluctuations and lowering the long-term average of blood glucose with pharmaceutical intervention in fact has almost no impact on the incidence of complications. This is not good news for patients with diabetes who obviously have reason to be seriously concerned about the need to prevent complications.
Standard vs. Intensive glucose control
There has been great interest in intensive blood glucose control and the natural (or perhaps simplistic) assumption has been that lowering levels to the pre-diabetic range with multiple drugs, insulin and dose escalation would be beneficial. The wake-up call came in 2008. Two papers simultaneously published in the New England Journal of Medicine reported on studies designed to address this issue with large cohorts. The ACCORD study randomized 10,251 patients with type 2 diabetes to receive intensive therapy targeted with polypharmacy, increased doses and insulin if necessary, all directed at getting HbA1c down from a median level of 8.1% to below 6.0%, generally considered high-normal or pre-diabetic. The controls received standard drug therapy targeting a level of 7.0% to 7.9%. As compared with standard therapy, the use of intensive therapy for 3.5 years increased mortality resulting in early trial termination and did not significantly reduce major cardiovascular events (Gerstein 2008).
The ADVANCE study randomized 11,140 patients with type 2 diabetes to either standard glucose control using mostly oral glycemic drugs or an intensive intervention group mainly using the modified release sulfonylurea Gliclazide, plus other drugs including insulin if needed to achieve a HbA1c of 6.5%. For over a median five- year follow-up, there was a small (1.9%) absolute decrease in risk associated with the primary endpoint, combined micro- and macrovascular events, in the intensive treatment group. But this was due to new or worsening nephropathy. Among 14 secondary endpoints, only new-onset miroablinuria showed a decreased absolute risk of 2%. Thus there was no impact on the remaining common complications of type 2 diabetes including all cause mortality, major or all coronary events, all cerebrovascular events, peripheral vascular events, visual deteriation or new or worsening neuropathy (Patel 2008).
A smaller trial, the Veterans Affairs Diabetes Trial (VADT) that reported in 2009, involved a comparison between intensive and standard glucose control in patients with type II diabetes with suboptimal response to therapy. In this study 1791 men, mean age 60, were randomized to two groups with a median follow-up of 5.6 years. Median HbA1c dropped from 8.4% in the standard therapy group to 6.9% in the intensive group. The primary endpoint was the time from randomization to first occurrence of a major cardiovascular event, which was a composite of MI, stroke, deaths from cardiovascular causes, congestive heart failure, surgery for vascular disease, inoperable coronary disease and amputation for gangrene. The comparison was with standard drug therapy. It was found that intensive glucose control had no significant impact on the endpoints of major cardiovascular events, death or microvascular complications with the exception of evidence of the progression to kidney disease. Statistically significant increased incidence of nephropathy was seen in subgroup analysis when micro- and macroalbuminuria were combined (Duckworth 2009).
In 2011 a systematic review and meta-analysis of randomized trials concerning intensive glycemic control appeared in BMJ (Hemmingsen 2011). No impact on all cause mortality was found. With regard to other endpoints, the authors found that data available remained insufficient to prove or refute a relative risk reduction of CVD mortality, non-fatal MI, composite microvascular complications, or retinopathy at a relative risk reduction threshold they considered clinically significant, but identified a 30% increase in severe hypoglycemia.
Prompted by the ADVANCE and VADT results and earlier studies related to kidney complications, a study involving meta-analysis reporting in 2012 investigated if intensive vs. standard glucose control decreased significant renal clinical outcomes such as doubling of serum creatinine levels, end-stage renal disease or death from renal disease during the years of follow-up. No evidence was found when seven trials with follow-up from two to 15 years were evaluated (Coca 2012).
Metformin monotherapy
The standard care almost always involves either metformin alone or metformin plus another drug. Since the American Diabetes Association (ADA) recommends metformin as the first-line of standard care after diagnosis of type 2 diabetes (ADA 2012), to what extent does it reduce the macro- and microvascular complications of diabetes when compared with a placebo or non-drug treatment such as diet? This may seem like an odd issue bring up. Metformin has been used for decades. Metformin is also the most common standard against which other drugs are judged in clinical trials.
In 2012 a meta-analysis was published where the efficacy of metformin was examined. The trials were either metformin vs. diet or non-drug care or a placebo, metformin plus a sulphonylurea vs. the sulphonylurea alone, or metformin plus insulin vs. insulin plus a placebo or metformin vs. total withdrawal from the drug. For the above comparisons, no significant benefit was found for all-cause mortality, cardiovascular mortality, all MI, all strokes, congestive heart failure, peripheral vascular events, amputation, or microvascular events (Boussageon 2012).
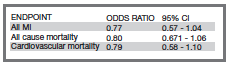
It is also important to look at just the studies in the above meta-analysis where metformin alone was compared with a placebo, diet or usual non-drug care and there were more than a few events. There are only two studies with longer than 12 months follow-up (Rachmani, 2002, UKPDS-34 1998), and only the endpoints of all cause mortality, cardiovascular mortality and all MI (heart attacks) were common to these studies. A third study lasting only 12 months was not judged relevant but was in fact negative (Cryer 2005). If the results from the two eligible studies are pooled and given equal weight (in the above meta-analysis they were given approximately equal weight), then as shown in the table below, for the three endpoints there was no statistically significant benefit found (95% confidence interval contains 1.0) These two studies together involved 537 subjects in the treated group and 609 in the control group.
Pooled analysis of two trials of metformin vs. diet (UKPDS-34 1998) or metformin withdrawal (Rachmani 2002).
The withdrawal study obtained only non-significant results indicating no benefit. The UKPDS-34 study is generally the only trial cited to justify the use of metformin because it claimed to have obtained evidence of benefit (UKPDS-34 1998). This study actually started in 1983 and reported finally in 1998. Over this period, the primary and main endpoints were changed a number of times and the announced termination date repeatedly extended. Furthermore, the study was not blinded for the investigators and the results of interim analysis were available. This study protocol is obviously open to bias. To quote one citric, “It seems that the authors continued the study until they obtained a result that was significant without adjusting for repeatedly looking at the data” (Ewart 2001). An investigator involved in the trial commented in 2008 that “UKPDS-34 broke almost all the rules of trial design. We are taught to believe that a study protocol should be predetermined and set in stone, but this study went to the other extreme, elevating the ad hoc into an art form” (Gale 2008). Thus the key study appears flawed.
In 2009 it was pointed out (Reaven 2009), based on the six-year report from UKPDS (UKPDS-16 1995), that following enrolment there was a progressive deteriation of glycemic control in all groups including those assigned to intensive control. In addition, beta cell function was estimated by HOMA-β and the investigators concluded that increasing hyperglycemia and decreasing beta cell function were significant features irrespective of the drug protocol used. Reaven also points out that the loss of secretory function does not appear to be inexorable and cites two studies where weight loss improved glycemic control. This view is supported by a study discussed below (Lim 2011).
The 2012 American Diabetes Association (ADA) Standards of Medical Care in Diabetes clearly indicates that metformin should be initiated as therapy for type 2 diabetes at the time of diagnosis. If the patient has markedly elevated blood glucose or HbA1c, then the advice is to consider insulin with or without oral agents. If metformin alone does not achieve or maintain satisfactory HbA1c levels over three to six months at maximal tolerated doses, the recommendation is to add a second oral agent (ADA 2012). This more intensive treatment is of course the subject of the several major studies discussed above which in general found no benefit. Furthermore, in the intensive vs. standard glucose lowering studies, metformin, the common reference treatment, itself does not appear to be effective. It is in fact quite remarkable that UKPDS-34 is the study used to justify metformin as the primary treatment and the drug used as a principal standard against which other drugs are tested.
Discussion and conclusions
There appears to be only very weak if any evidence regarding the ability of current drug treatments to significantly reduce the incidence of the many complications of type 2 diabetes, independent of the HbA1c level achieved. Among some diabetes researchers this is acknowledged and is termed the Diabetes Paradox, i.e. hyperglycemia is necessary for the development of late diabetic complications but average blood glucose (HbA1c) explains only a small percentage of complications (Bierhause 2011). Concerned researchers are now looking into the complex details of type 2 diabetes such as for example the potential for reactive glucose metabolites and their intermediates to act as active agents in the disease etiology and progression (Fleming 2012). There is obviously an urgent need for a new treatment paradigm rather than to merely reduce markers such as HbA1c.
In the past few years there have been calls (from the wilderness) for a return to the traditional approach used for decades for treating type 2 diabetes, the adoption of carbohydrate restriction in the context of both prevention and therapy (Accurso 2008, Leite 2009, Volek 2005a, Volek 2005b, Volek 2008, Volek 2009). However, such proposals face strong opposition from those who fear the substitution of fat for carbohydrates, even though there is compelling literature indicating that such fears are groundless (Ware 2012). The success achieved by Richard K. Bernstein using carbohydrate restriction and carefully selected carbohydrates matched to the individual’s metabolism is now documented in several editions of his book Dr. Bernstein’s Diabetes Solution (Bernstein 2011). The HbA1c levels he achieves are significantly better than anything conventional medicine appears able to accomplish, and this is generally only with diet. In fact, a recent study found complete reversal of type 2 diabetes achieved over a short period by a starvation diet, and
the benefits appear durable after return to higher calorie intake, strongly suggesting beta-cell regeneration and a permanent increase in insulin sensitivity (Lim 2011).
Are type 2 diabetes patients being given a false sense of security? They are told they need to get their blood glucose under control and many attempt to accomplish this in the belief that this reduces the risk of complications, which does not seem to be the case. The net result is a missed opportunity to restrict carbohydrates and calories and thus address the role of insulin in both insulin resistance, fat storage, and leptin resistance, deal with obesity and the metabolic syndrome, and also build muscle with resistance exercise to help increase insulin sensitivity. Another missed opportunity involves alternative medicines such as curcumin, which has produced better glucose control than prescription drugs and even appears to have reversed type 2 diabetes (Chuengsamarn 2012, Na 2012). Those who argue, probably with some justification, that severe carbohydrate restriction is not practical and adherence nearly impossible are also admitting that progress in reducing the complications of diabetes is at present nearly hopeless.
Reference List
Accurso,A., Bernstein,R.K., Dahlqvist,A., Draznin,B., Feinman,R.D., Fine,E.J., Gleed,A., Jacobs,D.B., Larson,G., Lustig,R.H., Manninen,A.H., McFarlane,S.I., Morrison,K., Nielsen,J.V., Ravnskov,U., Roth,K.S., Silvestre,R., Sowers,J.R., Sundberg,R., Volek,J.S., Westman,E.C., Wood,R.J., Wortman,J. and Vernon,M.C. Dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: time for a critical appraisal. Nutr Metab (Lond) 2008; 5: 9.
ADA. Standards of medical care in diabetes–2012. Diabetes Care 2012; 35 Suppl 1: S11-S63.
Bernstein,R.K., 2011. Dr. Bernstein’s Diabetes Solution. The complete guide to acheiving normal blood sugars. Little, Brown and Company, New York.
Bierhause,A., 2011. Failure of glucose lowering in clinical trials: The way to novel biochemical concedpts explaining diabetic late complications.The 26th Camillo Golgi Lecture, European Association for the Study of Diabetes. Annual meeting, Lisbon, September 2012.
Boussageon,R., Supper,I., Bejan-Angoulvant,T., Kellou,N., Cucherat,M., Boissel,J.P., Kassai,B., Moreau,A., Gueyffier,F. and Cornu,C. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomised controlled trials. PLoS Med 2012; 9(4): e1001204.
Chuengsamarn,S., Rattanamongkolgul,S., Luechapudiporn,R., Phisalaphong,C. and Jirawatnotai,S.
Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012; 35(11): 2121-2127.
Coca,S.G., Ismail-Beigi,F., Haq,N., Krumholz,H.M. and Parikh,C.R. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med 2012; 172(10): 761-769.
Cryer,D.R., Nicholas,S.P., Henry,D.H., Mills,D.J. and Stadel,B.V. Comparative outcomes study of metformin intervention versus conventional approach the COSMIC Approach Study. Diabetes Care 2005; 28(3): 539-543.
Duckworth,W., Abraira,C., Moritz,T., Reda,D., Emanuele,N., Reaven,P.D., Zieve,F.J., Marks,J., Davis,S.N., Hayward,R., Warren,S.R., Goldman,S., McCarren,M., Vitek,M.E., Henderson,W.G. and Huang,G.D. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360(2): 129-139.
Ewart,R.M. The case against aggressive treatment of type 2 diabetes: critique of the UK prospective diabetes study. BMJ 2001; 323(7317): 854-858.
Fleming,T., Cuny,J., Nawroth,G., Djuric,Z., Humpert,P.M., Zeier,M., Bierhaus,A. and Nawroth,P.P. Is diabetes an acquired disorder of reactive glucose metabolites and their intermediates? Diabetologia 2012; 55(4): 1151-1155.
Gale,E.A. Glucose control in the UKPDS: what did we learn? Diabet. Med 2008; 25 Suppl 2: 9-12.
Gerstein,H.C., Miller,M.E., Byington,R.P., Goff,D.C., Jr., Bigger,J.T., Buse,J.B., Cushman,W.C., Genuth,S., Ismail-Beigi,F., Grimm,R.H., Jr., Probstfield,J.L., Simons-Morton,D.G. and Friedewald,W.T. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358(24): 2545-2559.
Hemmingsen,B., Lund,S.S., Gluud,C., Vaag,A., Almdal,T., Hemmingsen,C. and Wetterslev,J. Intensive glycaemic control for patients with type 2 diabetes: systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. BMJ 2011; 343: d6898.
Leite,J.O., DeOgburn,R., Ratliff,J.C., Su,R., Volek,J.S., McGrane,M.M., Dardik,A. and Fernandez,M.L. Low-carbohydrate diet disrupts the association between insulin resistance and weight gain. Metabolism 2009; 58(8): 1116-1122.
Lim,E.L., Hollingsworth,K.G., Aribisala,B.S., Chen,M.J., Mathers,J.C. and Taylor,R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011; 54(10): 2506-2514.
Na,L.X., Li,Y., Pan,H.Z., Zhou,X.L., Sun,D.J., Meng,M., Li,X.X. and Sun,C.H. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: a double-blind, placebo-controlled trial. Mol Nutr Food Res 2012.
Patel,A., MacMahon,S., Chalmers,J., Neal,B., Billot,L., Woodward,M., Marre,M., Cooper,M., Glasziou,P., Grobbee,D., Hamet,P., Harrap,S., Heller,S., Liu,L., Mancia,G., Mogensen,C.E., Pan,C., Poulter,N., Rodgers,A., Williams,B., Bompoint,S., de Galan,B.E., Joshi,R. and Travert,F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358(24): 2560-2572.
Rachmani,R., Slavachevski,I., Levi,Z., Zadok,B., Kedar,Y. and Ravid,M. Metformin in patients with type 2 diabetes mellitus: reconsideration of traditional contraindications. Eur J Intern Med 2002; 13(7): 428.
Reaven,G.M. HOMA-beta in the UKPDS and ADOPT. Is the natural history of type 2 diabetes characterised by a progressive and inexorable loss of insulin secretory function? Maybe? Maybe not? Diab. Vasc Dis Res 2009; 6(2): 133-138.
UKPDS-16 U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes 1995; 44(11): 1249-1258.
UKPDS-34. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352(9131): 854-865.
Volek,J.S. and Feinman,R.D. Carbohydrate restriction improves the features of Metabolic Syndrome. Metabolic Syndrome may be defined by the response to carbohydrate restriction. Nutr Metab (Lond) 2005a; 2: 31.
Volek,J.S., Fernandez,M.L., Feinman,R.D. and Phinney,S.D. Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog. Lipid Res 2008; 47(5): 307-318.
Volek,J.S. and Forsythe,C.E. The case for not restricting saturated fat on a low carbohydrate diet. Nutr Metab (Lond) 2005b; 2: 21.
Volek,J.S., Phinney,S.D., Forsythe,C.E., Quann,E.E., Wood,R.J., Puglisi,M.J., Kraemer,W.J., Bibus,D.M., Fernandez,M.L. and Feinman,R.D. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009; 44(4): 297-309.
Ware,W.R. Saturated fat. Friend, foe or simply neutral. Integrated Healthcare Practitioners 2012; 5(2): 68.