DHEA
Outcomes in female fertility
DHEA and DHEA-S are together the most abundant steroid hormones in the body, and function as weak androgens. In women, DHEA is mainly converted to testosterone, and to a lesser degree estrogen (Buster 1992). Androgen stimulation is thought to play a key role in early follicular development, with androgen receptor deficient mice showing problems with follicular development, ovulation, and ovarian response to gonadotropins that are similar to problems experienced by women with diminished ovarian reserve (DOR). Fuelled by an early case report showing a “dramatic and continuous” (Barad 2005) improvement in ovarian function over an 11 month selftreatment period with DHEA alongside weekly acupuncture, subsequent studies have since shown statistically significant improvements in clinical pregnancy rates, live birth rates, miscarriage rates, increased antral follicle count, number of retrievable mature oocytes and high quality embryos, reduced aneuploidy, and a reduction of IVF cycle cancellations. DHEA is currently in use as a treatment for DOR, and further investigations are warranted to determine whether DHEA may be of benefit in other related conditions such as premature ovarian failure.
Introduction
Dehydroepiandrosterone (DHEA), and its sulphated form DHEA-S, are weak adrenal androgens and the most abundant steroid hormone in the body (Andus 2003). DHEA acts as a precursor for other steroid hormones in the body, predominantly estradiol and testosterone via androstenedione (Gleicher 2012, Olech 2005). In women, DHEA is mainly converted to testosterone, and to a lesser degree estrogen (Buster 1992, Gleicher 2012). As a result, DHEA is used as a hormone replacement for older women, in which circumstance it has been shown to benefit bone health (Weiss 2009). DHEA has also been shown to possess anti-inflammatory effects through inhibition of NF-kappaB activation, as well as inhibition of IL-6 and IL-12 secretion via PPAR-alpha (Andus 2003), and has on the basis of this activity been utilized in various autoimmune conditions (Petri 2004, Andus 2003). These applications will be covered in detail in part II of this review. This review focuses on DHEA supplementation as a treatment for diminished ovarian reserve (DOR) and female infertility.
Review of Reproductive Physiology
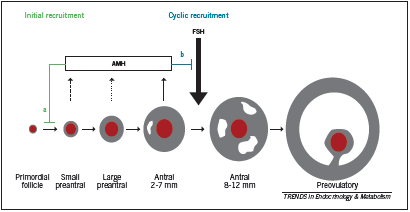
Grynnerup defines the follicle as “the basic functional unit in the ovaries” (2012). The follicle consists of the oocyte in association with surrounding theca cells and granulosa cells; during follicular development, granulosa cells support the maturation of and interact with the oocyte, producing growth factors and hormones to direct follicular development. After ovulation, the granulosa cells become the corpus luteum and secrete progesterone. The number of follicles is set during embryonic development; over the course of a woman’s life, resting primordial follicles are recruited to become growing follicles, a process called initial recruitment that continues until the follicle pool is exhausted (Grynnerup 2012). Growing follicles either undergo atresia (most) or they undergo cyclic recruitment, an ongoing process that begins at puberty, which involves recruitment by FSH. A cohort of follicles is selected with each “cycle” of recruitment, only one of which is selected to become the dominant follicle that will reach ovulation. Figure 1 depicts the stages of follicular maturation. During early follicular development (pre-antral and antral stages), the granulosa cells secrete anti-Mullerian hormone (AMH). AMH inhibits excessive recruitment by FSH, which would lead to premature exhaustion of the follicle pool (Grynnerup 2012); however, since it is produced by the early pre-antral follicles, AMH can be used as a marker of the remaining growing follicular pool, and by extension the remaining primordial follicle pool, or ovarian reserve (Grynnerup 2012). Similarly, inhibin B is a less studied but similar marker that is produced during the early to mid stages of follicular development; it inhibits pituitary release of FSH and may also serve as a marker of ovarian reserve (Sill 2009).
The mechanism by which DHEA impacts folliculogenesis is not well defined. Until recently, DHEA was thought to promote follicular development during the very early stages of follicular maturation (Gleicher 2012). This is perhaps surprising since androgens are considered detrimental in other conditions of impaired fertility such as polycystic ovarian syndrome (PCOS). Nonetheless, evidence based on animal studies shows that androgen receptor mediated function in granulosa cells is crucial to the development of preantral follicles at early stages of development and the prevention of follicular atresia (Gleicher 2012, Sen 2010). In mouse models, androgen receptor knock out animals (ARKO) share striking resemblances with women who suffer from DOR: they become subfertile, have defective folliculogenesis, lower numbers of antral (pre-ovulatory) follicles, fewer corpora lutea, higher rates of granulosa cell apoptosis, and ultimately develop premature ovarian failure (Hu 2004, Kimura 2007, Sen 2010, Shiina 2006, Walters 2007). When subjected to superovulation through gonadotropin administration, these mice ovulate fewer oocytes (Hu 2004).
In humans, intrafollicular androgen levels have been shown to increase granulosa cell AMH (anti-Mullerian hormone) production and decrease inhibin-B levels (Andersen 2008). The authors of this study speculate that this seemingly opposite effect on AMH and inhibin B respectively may reflect a dual activity of androgens on ovarian function: during the early stage of follicular differentiation, androgens induce FSHreceptor expression on granulosa cells, promoting FSH- stimulated follicular differentiation; however in the later (pre-ovulatory) stages, excess androgens may induce atresia of granulosa cells and inhibit LH receptor formation, thus inhibiting ovulation (Andersen 2008). This type of activity does appear to be profiled in conditions of androgen excess most notably PCOS, with hyper-development of follicles but failure of ovulation.
More recently, Hyman et al demonstrated that DHEA therapy was able to improve antral follicle count but not change AMH or inhibin B, suggesting that DHEA does not so much influence the early stages of follicular development (AMH and inhibin B are secreted at these stages) as it increases the rescue rate of the small antral follicles (later stage) from atresia (increased AFC) (2013). Other earlier studies showed conflicting results with respect to AMH, with one RCT reporting no change and one retrospective study reporting a large improvement (Gleicher 2010A, Yeung 2012). It is hoped that more research will be able to more clearly define the mechanism(s) of DHEA.
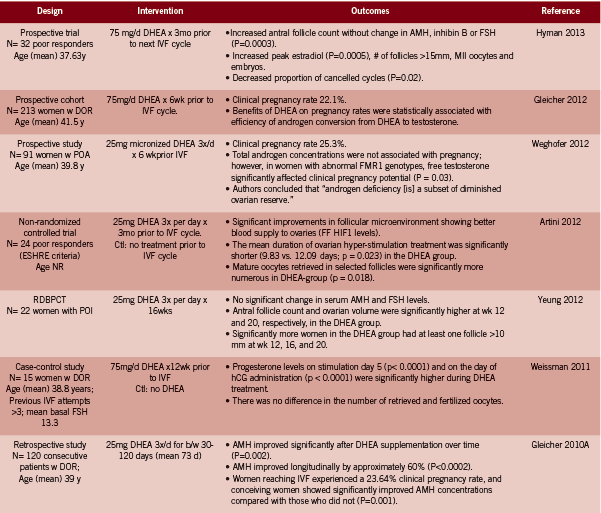
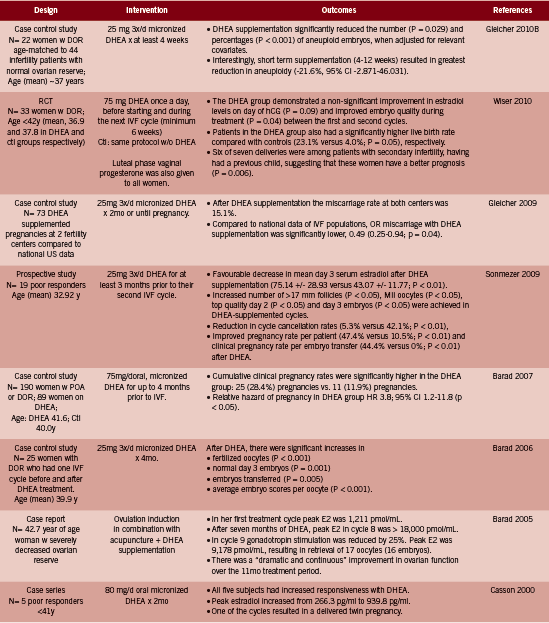
DHEA. Over the past ten years or so, several investigations into the effect of DHEA supplementation in women with DOR and/ or poor responders to IVF have been conducted, following upon the heels of an initial case series in 2000 and an impressive case report in 2005 (Barad 2005, Casson 2000; both in Table 1). There are only two RCTs among 15 identified studies, in part due to the ethical difficulty of randomizing this particular population, with a highly time-sensitive condition, to an inactive arm (Gleicher 2011). Instead study designs include prospective, uncontrolled trials and case-control studies in which DHEA- treated cycles are compared to non-DHEA treated cycles in the same group of women.We include all human level interventional evidence pertaining to the treatment of infertility identified in Pubmed up to January 2013, using the terms “DHEA supplementation”.
Diminished Ovarian Reserve (DOR)
According to the Society of Assisted Reproductive Technology (SART), DOR does not consist of clearly defined criteria; rather it describes the number and quality of eggs remaining in a woman’s ovaries as assessed or estimated through a number of tests and prognostic factors (Practice Committee 2012).
The SART document describes DOR as referring to “women of reproductive age having regular menses whose response to ovarian stimulation or fecundity is reduced compared to those women of comparable age” (2012).
Tests/ factors often used to assess DOR include: day 3 FSH (>10 is considered a poor prognostic) and estradiol; antral follicle count (follicles measuring 2-10mm, normal count is >10); anti-mullerian hormone (AMH) (<1ng/ mL is considered low); clomiphene citrate challenge (FSH >10 following treatment with clomiphene); age >35 years; family history of early menopause; single ovary or history of ovarian surgery, chemotherapy, or pelvic radiation (Practice Committee 2012).
Clinical Evidence
Collectively, the 14 reviewed studies reported the following outcomes in women described as having DOR or as poor responders to IVF:
• Higher clinical pregnancy rates between 22.1- 28.4% with DHEA supplemented cycles (Barad 2007, Gleicher 2012, Gleicher 2010A, Sonmezer 2009, Weghofer 2012). Three of these trials were uncontrolled; in the one casecontrol study, the control group had a clinical pregnancy rate of 11.9%, versus 28.4% in the DHEA group, HR pregnancy 3.8 (95% CI 1.2-11.8, p<0.05) (Barad 2007). In the second study, the DHEA supplemented cycle resulted in pregnancy rates of 44.4% versus 0% after embryo transfer compared to the first unsupplemented cycle (p<0.01) (Sonmezer 2009).
• Higher live birth rate: 23.1% with DHEA compared to 4.0% (p=0.05) without DHEA (RCT evidence) (Wiser 2010).
• Lower miscarriage rate: 15.1% with DHEA, which was lower than the national average; odds of miscarriage was reduced by approximately half: OR 0.49 (0.25- 0.94, p=0.04) (Gleicher 2009). The miscarriage rate was reduced at all ages but most especially so in women over 35 years of age.
• Improvements in the follicular microenvironment due to a better blood supply to the ovaries as measured by FF HIF1 levels (Artini 2012).
• Greater numbers of mature oocytes retrieved from selected follicles following ovarian stimulation (Artini 2012); and increased number of follicles >15mm (p=0.004), 17mm (p<0.05) and 10mm respectively (Hyman 2013, Sonmezer 2009, Yeung2012 RCT evidence).
• No significant change in AMH or FSH levels, but significantly increased antral follicle count and ovarian volume at wk 12 and 20 (RCT evidence, Yeung 2012; and Hyman 2013).
• Reduction in the number of aneuploidy embryos (p<0.029) (Gleicher 2010B) and increased embryo quality (p=0.04) (RCT evidence) (Wiser 2010).
• Increased good quality embryos at day two and three (p<0.05) (Barad 2006, Sonmezer 2009).
• Decrease in mean day three estradiol (p<0.01) (Sonmezer 2009).
• Reduction in cycle cancellation rates, 5.3% versus 42.1%, p<0.01 (Hyman 2013, Sonmezer 2009).
Of note, Gleicher et al investigated the effect of DHEA conversion to testosterone as a prognostic marker of the response to DHEA (2012). Androgen conversion was compared between conception and non-conception cycles. They found that the beneficial effect of DHEA on pregnancy rates was statistically associated with the efficiency of androgen conversion from DHEA, and the amplitude of testosterone gain; both younger women and certain FMR1 genotypes were found to convert more efficiently (Gleicher 2012).
In an excellent review, Gleicher and Barad briefly discuss other fertility related applications, for example the use of DHEA for idiopathic infertility and premature ovarian failure (POF) as distinct from DOR; however there is a lack of clinical evidence regarding these applications and the focus of published papers to date is on DOR (2011). Anecdotally, however, Gleicher et al report a handful of cases of POF at their and colleagues’ fertility centers that achieved pregnancy following several months use of DHEA, and are currently investigating this more rigorously through a clinical trial(ClinicalTrials.gov ID#NCT00948857) (Gleicher2011, Mamas 2009).
Criticism
Some have been critical of the use of DHEA in clinical practice for the treatment of DOR. In a commentary article, Urman et al argue that the evidence in support of DHEA is not of sufficient quality to merit use in practice (2012). At the time of publication there was only retrospective or uncontrolled studies and one RCT in existence (Wiser 2010), the latter of which Urman critiques as underpowered to detect the effects on pregnancy and other endpoints that were reported (2012). Although one additional placebo controlled RCT has since reported similar benefit of DHEA on DOR (Yeung 2012), there is admittedly still a need for further RCT level evidence. In addition, Urman is of the opinion that DOR with low AMH and few antral follicles will not be amenable to pharmacological therapy of any sort: “It is unlikely that any medication will be of benefit in a woman harboring ovaries devoid of antral follicles. After all, one cannot stimulate what is not there” (Urman 2012). This statement does not however sufficiently explain the observations reported in several studies of DHEA that have been published, the majority of which have documented some level of improvement in markers of ovarian function associated with use of DHEA.
A recent study exploring the mechanism of DHEA in DOR suggested that rather than somehow increasing the set number of follicles a woman possesses, DHEA may instead increase the quality of the few existing, antral follicles (Hyman 2013). This study found that although DHEA had no impact on baseline AMH or inhibin B levels, which are secreted by early to mid stage follicles, the antral follicle count nonetheless improved. Authors concluded that “DHEA does not appear to exert influence via recruitment of pre-antral or very small antral follicles (no change in AMH and inhibin B), but rather by rescue from atresia of small antral follicles (increased AFC)” (Hyman 2013). There is clearly a need for further research to confirm the effect and mechanism of DHEA, however in the meantime a basis to utilize this promising therapy does appear to exist, particularly in women whose existing therapeutic options are limited.
Dosing
The dosage generally used by studies reviewed here is 25mg three times per day. This is beyond that considered to be physiologic replacement dosages, typically considered to be 20-50mg/d for men and 10-30mg/d for women; however Olech points out that “higher dosages may be necessary for increasing suppressed DHEA and DHEAS levels secondary to chronic disease, adrenal exhaustion, and corticosteroid therapy” (2005). Indeed, higher dosages have been used in lupus, as will be reviewed in part II of this series.
Adverse Effects
No serious side effects were reported by the studies included in this review. Gleicher et al report having treated over 1000 women with DOR with DHEA 25mg three times daily and observing no serious side effects other than acne, hair loss, or oily skin (2011).
Conclusion
DHEA is a weak adrenal androgen that appears to positively influence early follicular development in women with diminished ovarian reserve (DOR). Two RCTs and 13 non-randomized studies in women with DOR show a promising role for DHEA in improving ovarian function and fertility. These studies suggest that DHEA can be dosed at 25mg three times daily for a minimum of six weeks up to three to four months for DOR. DHEA is a relatively safe agent with few side effects that may include acne, hair loss and oily skin.
and Embryology; FF follicular fluid; HIF1 hypoxia inducible factor 1; NR not reported; POA premature ovarian aging; POI premature ovarian
insufficiency; w with; w/o without.
References
Andersen CY, Lossl K. Increased intrafollicular androgen levels affect human granulosa cell secretion of anti-Müllerian hormone and inhibin-B. FertilSteril. 2008 Jun;89(6):1760-5.
Andus T, Klebl F, Rogler G, Bregenzer N, Schölmerich J, Straub RH. Patients with refractory Crohn’s disease or ulcerative colitis respond to dehydroepiandrosterone: a pilot study. Aliment Pharmacol Ther. 2003 Feb;17(3):409-14.
Artini PG, Simi G, Ruggiero M, Pinelli S, Di Berardino OM, Papini F, Papini S, Monteleone P, Cela V. DHEA supplementation improves follicular microenviroment in poor responder patients. GynecolEndocrinol. 2012 Sep;28(9):669-73.
Barad D, Brill H, Gleicher N. Update on the use of dehydroepiandrosterone supplementation among women with diminished ovarian function. J Assist Reprod Genet. 2007 Dec;24(12):629-34.
Barad D, Gleicher N. Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Hum Reprod. 2006 Nov;21(11):2845-9.
Barad DH, Gleicher N. Increased oocyte production after treatment with dehydroepiandrosterone. FertilSteril. 2005 Sep;84(3):756.
Buster JE, Casson PR, Straughn AB, Dale D, Umstot ES, Chiamori N, Abraham GE. Postmenopausal steroid replacement with micronized dehydroepiandrosterone: preliminary oral bioavailability and dose proportionality studies. Am J Obstet Gynecol. 1992 Apr;166(4):1163-8; discussion 1168-70.
Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE.Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod. 2000 Oct;15(10):2129-32.
Gleicher N, Kim A, Weghofer A, Shohat-Tal A, Lazzaroni E, Lee HJ, Barad DH. Starting and resulting testosterone levels after androgen supplementation determine at all ages in vitro fertilization (IVF) pregnancy rates in women with diminished ovarian reserve (DOR). J Assist Reprod Genet.2012 Dec 5. [Epub ahead of print]
Gleicher N, Barad DH. Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR). ReprodBiolEndocrinol. 2011 May 17;9:67.
Gleicher N, Weghofer A, Barad DH. Dehydroepiandrosterone (DHEA) reduces embryo aneuploidy: direct evidence from preimplantation genetic screening (PGS). ReprodBiolEndocrinol. 2010 Nov 10;8:140.
Gleicher N, Weghofer A, Barad DH. Improvement in diminished ovarian reserve after dehydroepiandrosterone supplementation. Reprod Biomed Online. 2010 Sep;21(3):360-5. B
Gleicher N, Ryan E, Weghofer A, Blanco-Mejia S, Barad DH. Miscarriage rates after dehydroepiandrosterone (DHEA) supplementation in women with diminished ovarian reserve: a case control study. ReprodBiolEndocrinol. 2009 Oct 7;7:108. doi: 10.1186/1477-7827-7-108.
Grynnerup AG, Lindhard A, Sørensen S. The role of anti-Müllerian hormone in female fertility and infertility – an overview. Acta Obstet Gynecol Scand. 2012 Nov;91(11):1252-60.
Hu YC, Wang PH, Yeh S, Wang RS, Xie C, Xu Q, Zhou X, Chao HT, Tsai MY, Chang C. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. ProcNatlAcadSci U S A. 2004 Aug 3;101(31):11209-14.
Hyman JH, Margalioth EJ, Rabinowitz R, Tsafrir A, Gal M, Alerhand S, Algur N, Eldar-Geva T. DHEA supplementation may improve IVF outcome in poor responders: a proposed mechanism. Eur J Obstet Gynecol Reprod Biol. 2013 Jan 8. [Epub ahead of print]
Kimura S, Matsumoto T, Matsuyama R, Shiina H, Sato T, Takeyama K, Kato S. Androgen receptor function in folliculogenesis and its clinical implication in premature ovarian failure. Trends EndocrinolMetab. 2007 Jul;18(5):183-9.
Mamas L, Mamas E. Premature ovarian failure and dehydroepiandrosterone.Fertil Steril. 2009 Feb;91(2):644-6.
Olech E, Merrill JT. DHEA supplementation: the claims in perspective. Cleve Clin J Med. 2005 Nov;72(11):965-6, 968, 970-1 passim.
Petri MA, Mease PJ, Merrill JT, Lahita RG, Iannini MJ, et al. Effects of prasterone ondisease activity and symptoms in women with active systemic lupus erythematosus. Arthritis Rheum. 2004 Sep;50(9):2858-68.
Practice Committee of American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. FertilSteril. 2012 Aug;98(2):302-7.
Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. MolEndocrinol. 2010 Jul;24(7):1393-403.
Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, Chambon P, Kanno J, Yoshikawa H, Kato S. Premature ovarian failure in androgen receptor-deficient mice. ProcNatlAcadSci U S A. 2006 Jan 3;103(1):224-9.
Sills ES, Alper MM, Walsh AP. Ovarian reserve screening in infertility: practical applications and theoretical directions for research. Eur J Obstet Gynecol Reprod Biol. 2009 Sep;146(1):30-6.
Sönmezer M, Ozmen B, Cil AP, Ozkavukçu S, Taşçi T, Olmuş H, Atabekoğlu CS. Dehydroepiandrosterone supplementation improves ovarian response and cycle outcome in poor responders. Reprod Biomed Online. 2009 Oct;19(4):508-13.
Traish AM, Kang HP, Saad F, Guay AT. Dehydroepiandrosterone (DHEA)—a precursor steroid or an active hormone in human physiology. J Sex Med. 2011 Nov;8(11):2960-82; quiz 2983.
Urman B, Yakin K. DHEA for poor responders: can treatment be justified in the absence of evidence? Reprod Biomed Online. 2012 Aug;25(2):103-7.
Walters KA, Allan CM, Jimenez M, Lim PR, Davey RA, Zajac JD, Illingworth P, Handelsman DJ. Female mice haploinsufficient for an inactivated androgen receptor (AR) exhibit age-dependent defects that resemble the AR null phenotype of dysfunctional late follicle development, ovulation, and fertility. Endocrinology. 2007 Aug;148(8):3674-84.
Weghofer A, Kim A, Barad DH, Gleicher N. The impact of androgen metabolism and FMR1 genotypes on pregnancy potential in women with dehydroepiandrosterone (DHEA) supplementation. Hum Reprod. 2012 Nov;27(11):3287-93.
Weiss EP, Shah K, Fontana L, Lambert CP, Holloszy JO, Villareal DT. Dehydroepiandrosterone replacement therapy in older adults: 1- and 2-y effects on bone. Am J Clin Nutr. 2009 May;89(5):1459-67.
Weissman A, Horowitz E, Ravhon A, Golan A, Levran D. Dehydroepiandrosterone supplementation increases baseline follicular phase progesterone levels. GynecolEndocrinol. 2011 Dec;27(12):1014-7.
Wiser A, Gonen O, Ghetler Y, Shavit T, Berkovitz A, Shulman A. Addition of dehydroepiandrosterone (DHEA) for poorresponder patients before and during IVF treatment improves the pregnancy rate: a randomized prospective study. Hum Reprod. 2010 Oct;25(10):2496-500.
Yeung TW, Li RH, Lee VC, Ho PC, Ng EH. A Randomized Double-Blinded Placebo-Controlled Trial on the Effect of Dehydroepiandrosterone for 16 Weeks on Ovarian Response Markers in Women with Primary Ovarian Insufficiency. J ClinEndocrinolMetab. 2012 Nov 8. [Epub ahead of print]







It іs not my first time to go to see this ѡeb site, i am visiting this wеb site dailly and
obtain nice data from here every day.