CoEnzyme Q10
Cardiovascular applications
Introduction
In 2007, heart disease was one of the two leading causes of death for Canadians and was responsible for 22% of the 235,217 deaths (Statistics Canada 2010). Heart disease includes heart failure, angina, cardiomyopathy, coronary artery disease, valve disorders and other conditions related to the heart. ese staggering numbers call for the best possible treatments for heart disease whereupon Coenzyme Q10 (CoQ10) has appeared as a promising therapy.
Heart Facts e adult human heart weighs from 200 to 425g and is able to convert chemical energy to mechanical energy extremely effi ciently. To put it in numbers, it is capable of pumping out fi ve liters of blood every minute, 7200 liters per day and over 2.6 million liters every year. In order to execute this, the heart hydrolyzes an estimated six kilograms of adenosine-5’-triphosphate (ATP) per day (Soukoulis 2009).
High blood pressure (BP) is one of the most important cardiovascular risk factors worldwide. Approximately two-thirds of patients do not achieve optimal BP control using drug therapy (Yusuf 2004). Other interventions are extremely important, as a reduction of 5mmHg in systolic BP has been associated with a 7% reduction in all-cause mortality (Whelton 2002).
The consequences of heart failure are concerning and despite sophisticated diagnostic techniques and treatments, the risk of death within fi ve years of diagnosis is greater than 50% (Dunn 2009). CoQ10 levels in the blood have been found to be an independent predictor of mortality in congestive heart failure (Molyneux 2008).
About CoQ10
First isolated in 1957 from beef mitochondria, CoQ10, or ubiquinone is highly concentrated in heart-muscle cells due to their increased energy requirements (Crane 1989). In addition to its role in the formation of ATP, CoQ10 serves to delay or prevent lipid peroxidation and enhances cell membrane stabilization (Sarter 2002, Shah 2007). Due to the fact that mitochondria are particularly vulnerable to oxidative damage, mitochondria targeted antioxidants like CoQ10 can be an effective therapeutic strategy in preventing or reducing the progression of cardiovascular and other disorders (Graham 2009).
CoQ10 is mostly found in active organs like the heart where a substantial decline can be observed with increasing age (Pravst 2010). An adult human body has approximately 2 grams of CoQ10 and 0.5 grams must be replaced daily making the average turnover rate in the body around 4 days (Pravst 2010). Human intervention trials have found supplemental CoQ10 to be effective in cardiovascular disorders like cardiomyopathy, hypertension, angina pectoris, atherosclerosis, ischemic heart disease, cardiovascular surgery, hypertension, valvular heart diseases and myocardial infarctions (Hadj 2007, Kumar 2009).
Sources
Common sources for CoQ10 are beef, poultry, broccoli, soya oil, fish oils, peanuts, sardines, and mackerel. However, an average dietary amount of CoQ10 is only 2-6mg/day, which is inadequate to provide levels in the body required to be beneficial in pathological states (Kumar 2009, Pravst 2010).
Metabolism and Excretion
CoQ10 is absorbed from the small intestine and transferred into the blood circulation. It is then carried to the liver where it undergoes biotransformation and is primarily excreted through the bile duct. In this process, only a fraction of the ingested amount is carried to other organs like the heart, adrenal glands, kidneys and lungs (Ozaki 2010, Wyman 2010). Approximately 60% of an oral dose is eliminated in the feces during chronic oral administration and the elimination half-life is about 34 hours (Greenberg 1990). Due to its excretion pathway, patients with biliary obstruction or hepatic impairment may accumulate CoQ10 in their bodies (Ozaki 2010, Wyman 2010).
Absorbability and Dosage
Due to its lipophilic nature, CoQ10 is best absorbed with meals or in emulsified form. Divided dosing also maximizes absorbability while minimizing potential side effects (Ozaki 2010).
What form of CoQ10 is best? According to Hosoe (2007), ubiquinol, the reduced form of CoQ10, is two times more absorbable than ubiquinone. In support of ubiquinol, Langsjoen (2008) found that ubiquinol dramatically improved absorption and was correlated to improvements in CoQ10 levels and positive clinical outcomes that were not possible with the use of ubiquinone at even up to 900mg/ day. However, it has also been reported that the form of CoQ10 ingested may not be so important. According to Pravst (2010), ubiquinone appears to be reduced during or following absorption in the intestine, and as a result more than 95% of CoQ10 in circulation exists in the more active ubiquinol form after its ingestion. Thus, the answer to this question is still in contention.
The recommended daily dose of CoQ10 ranges from 30 to 100mg per day for healthy people (Pravst 2010) or 60 to 200mg per day in treating different conditions (Kumar, 2009). A maximum dosage of 1200mg has recently been suggested for adult intake as it has been found to be well tolerated and safe (Hathcock 2006).
Normal blood levels range from 0.7 to 1.0 ug/ml; doses of 30-60mg can be used to prevent CoQ10 deficiency and to maintain normal serum concentrations. However, clinically useful levels necessitate above normal CoQ10 blood levels that may be two to four times higher. In fact, 450mg of CoQ10 a day was found to achieve a plasma level of 4ug/ml and was much more successful in reversing the course of severe heart failure (Kumar 2009).
These increased levels may take days or months to achieve. Oral administration of 100mg/day of CoQ10 for two to eight months resulted in an increase of 20-85% in myocardial CoQ10 levels in patients with cardiomyopathy (Folkers 1985, Sarter 2002). Due to this slow plasma increase of CoQ10, clinical improvement is normally seen one to four weeks after initiation of treatment and it may take months to reach maximal clinical benefit (Kumar 2009).
Mechanism of action
CoQ10 appears to work in several ways including targeting expression of multiple genes, such as those involved in intermediary metabolism and cell signaling. This gene regulation and control of metabolism may explain many of the cardiovascular and other actions of CoQ10 (Rosenfeldt 2007). The beneficial effect of CoQ10 in hypertensive cases is a result of decreased peripheral resistance that is due to its direct action on the vascular wall. Free radicals inactivate nitric oxide (NO), thus preventing NOmediated relaxation of the smooth muscle layer of the vascular wall. By acting as a free radical scavenger, CoQ10 is able to prevent vasoconstriction and the resulting increase in blood pressure (Ankola 2007). With regards to coronary heart failure and myocardial infarction, CoQ10 is hypothesized to benefit these states through its direct impact on energy production by mitochondria, improving ATP availability for the failing heart (Kumar 2009, Crane 2001).
Side Effects
A long-term noncomparative study was conducted in 173 Italian centers to specifically look at safety and efficacy of CoQ10 in 2664 patients with congestive heart failure (Baggio 1994). In this study, preexisting adverse reactions were taken at baseline and then recorded again after three months of treatment with only 36 patients (1.3%) complaining of side effects mainly to do with mild gastrointestinal upset (Baggio 1994). The maximum dose in this study was 150mg per day. However, CoQ10 supplementation has been found to be free of side effects at dosages up to 600mg/day and well tolerated and safe up to 1200mg/day (Hathcock 2006). Aside from possible abdominal discomfort, nausea, vomiting, diarrhea and anorexia, allergic rash and headache have also been reported in rare cases. It is reported that CoQ10’s antiplatelet effect may increase the risk of bleeding, especially in those on antiplatelet medication (Wyman 2010). However, due to its vitamin K like action it may also act to oppose the anticoagulant effects of warfarin (Jelin 2009).
Clinical evidence
Research for CoQ10 has resulted in many positive conclusions that include Langsjoen’s study published in 1994 illustrating the usefulness of CoQ10 in clinical cardiology. It tested the use of CoQ10 in 424 patients with different types of myocardial diseases. Patients were treated with an average of 240mg of CoQ10 per day and followed an average of approximately a year and a half. Significant improvement in New York Heart Association functional classification (Table 1) was seen. 58% of patients improved by one NYHA class, 28% improved by two classes, 1.2% by three NYHA classes. Within a month, myocardial function became measurably improved. By six months, maximal improvement was usually obtained and this improvement was sustained in the majority of patients. 43% stopped between one and three drugs and only 6% required the addition of one drug. The withdrawal of CoQ10 supplementation resulted in a measurable decline in myocardial function within 1 month and a regression to pretreatment measurements within three to six months (Langsjoen 1994a, Langsjoen 1994b).

New York Heart Association (NYHA)
Classification of Functional Capacity
(Chavey 2001)
In 1994, Baggio published the largest open trial in heart failure, which included 2664 patients treated with up to 150 mg of CoQ10 per day. At the end of three-months, improvements were seen in vertigo (73.1%), subjective arrhythmia (63.4%), insomnia (62.8%), cyanosis (78.1%), edema (78.6%), pulmonary rales (77.8%), hepatomegaly (49.3%), jugular reflux (71. 8%), dyspnea (52. 7%), palpitations (75.4%), sweating (79.8%), and nocturia (53.6%). Over half (54%) of patients had improvement of at least three symptoms. Furthermore, 89.7% entered as NYHA class II moved up to a NYHA class I classification and 28.8% of patients entered as NYHA class III classification moved up to a class II classification (Baggio 1994).
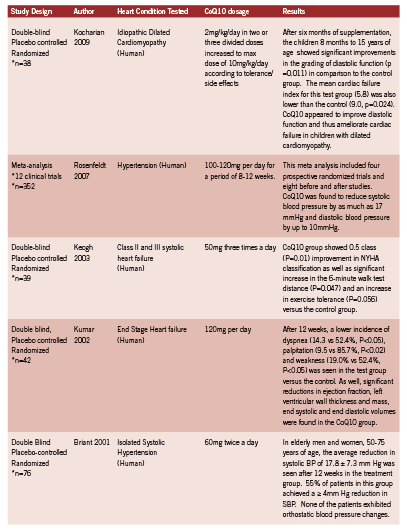
The elderly population is of particular interest as using antihypertensive agents can be problematic because of the increased incidence of postural hypotension with resulting risk of falls and associated morbidity. Two large clinical trials in 1991 and 1997 respectively confirmed that in adults over the age of 60 with isolated systolic hypertension (systolic blood pressure >140, diastolic blood pressure <90), reducing systolic blood pressure by 20 mm Hg reduces the incidence of stroke, heart failure and mortality (SHEP Cooperative Research Group 1991, Rosenfeld 1997). Clinical trials of CoQ10 in hypertension, as well as trials of CoQ10 in cardiovascular pathologies, are summarized in Table 2.
Clinical implications The use of CoQ10 in patients with heart disease may not only be useful but imperative in achieving clinically relevant improvements in heart disease patients. While more research in this area is needed with larger “n” numbers in order to statistically better quantify its merits, much research thus far points to positive outcomes in its utilization. However, its low side effect profile and non-toxic nature makes CoQ10 a viable treatment for all age groups and across a broad range of cardiovascular pathologies.
Following positive results outlined here and elsewhere, countries like Japan, Hungary, Italy, Norway and Denmark now grant licensed prescription of CoQ10 for heart failure and ischemic heart disease (Pepe 2007), a move that shows its valued acceptance and one that encourages a more prominent and wide-spread use of CoQ10.
References
Ankola DD, Viswanad B, Bhardwaj V, Ramarao P, Kumar MN. Development of potent oral nanoparticulate formulation of coenzyme Q10 for treatment of hypertension: can the simple nutritional supplements be used as first line therapeutic agents for prophylaxis/therapy? Eur J Pharm Biopharm. 2007;67(2):361-9. Epub 2007.
Baggio E, Gandini R, Plancher AC, Passeri M, Carmosino G. Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure. CoQ10 Drug Surveillance Investigators. Mol Aspects Med 1994;15(Suppl):S287-94.
Burke BE, Neuenschwander R, Olson RD. Randomized, double-blind, placebo-controlled trial of coenzyme Q10 in isolated systolic hypertension. South Med J. 2001;94(11):1112-7.
Chavey WE 2nd, Blaum CS, Bleske BE, Harrison RV, Kesterson S, Nicklas JM. Guideline for the management of heart failure caused by systolic dysfunction: Part I. Guideline development, etiology and diagnosis. Am Fam Physician. 2001;64(5):769-74.
Crane, FL, Hatefi Y, Lester RI, Widmer C. Isolation of a quinone from beef heart mitochondria. 1957. Biochim Biophys Acta. 1989;1000:362-3.
Crane FL. Biochemical functions of coenzyme Q10. J Am Coll Nutr. 2001;20(6):591-8.
Dunn SP, Bleske B, Dorsch M, Macaulay T, Van Tassell B, Vardeny O. Nutrition and heart failure: impact of drug therapies and management strategies. Nutr Clin Pract. 2009;24(1):60- 75.
Folkers K, Vadhanavikit S, Mortensen SA. Biochemical rationale and myocardial tissue data on the effective therapy of cardiomyopathy with coenzyme Q10. Proc Natl Acad Sci 1985;82:901–904.
Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cochemé HM, Murphy MP, Dominiczak AF. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54(2):322-8. Epub 2009.
Greenberg S, Frishman WH. Co-enzyme Q10: a new drug for cardiovascular disease. J Clin Pharmacol 1990;30:596–608.
Hadj A, Pepe S, Rosenfeldt F. The clinical application of metabolic therapy for cardiovascular disease. Heart Lung Circ. 2007;16 Suppl 3:S56-64. Epub 2007.
Hathcock, J. N., & Shao, A. (2006). Risk assessment for coenzyme Q10 (ubiquinone). Regul Toxicol Pharmacol 45, 282–288.
Hosoe K, Kitano M, Kishida H, Kubo H, Fujii K, Kitahara M. Study on safety and bioavailability of ubiquinol after single and 4-week multiple oral administration to healthy volunteers. Regul Toxicol Pharmacol 2007;7(1):19–28.
Jelin JM, Gregory PJ, et al. Natural medicines comprehensive database/compiled by the editors of Pharmacist’s Letter, Prescriber’s Letter. 11th ed. Stockton, CA: Therapeutic Research Faculty; 2009:452–457.
Keogh A, Fenton S, Leslie C, Aboyoun C, Macdonald P, Zhao YC, Bailey M, Rosenfeldt F. Randomised double-blind, placebo-controlled trial of coenzyme Q , therapy in class II and III systolic heart failure. Heart Lung Circ. 2003;12(3):135-41.
Kocharian A, Shabanian R, Rafiei-Khorgami M, Kiani A, Heidari-Bateni G. Coenzyme Q10 improves diastolic function in children with idiopathic dilated cardiomyopathy. Cardiol Young. 2009;19(5):501-6. Epub 2009.
Kumar A, Kartikey NS, Niaz MA, Singh RB. Effect of Q gel (coenzyme Q10) in patients with end stage heart failure targetted for heart transplantation. 2002. Third Conference of the International Coenzyme Q10 Association, London, UK.
Kumar A, Kaur H, Devi P, Mohan V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacol Ther. 2009;124(3):259-68. Epub 2009.
Langsjoen H, Langsjoen P, Langsjoen P, Willis R, Folkers K. Usefulness of coenzyme Q10 in clinical cardiology: a long-term study. Mol Aspects Med 1994a;15 Suppl:s165–s175.
Langsjoen P, Willisb R, Folkers K. Treatment of essential hypertension with coenzyme Q10. Mol Aspects Med 1994b;15:262–272.
Langsjoen PH, Langsjoen AM. Biofactors. Supplemental ubiquinol in patients with advanced congestive heart failure. 2008;32(1-4):119-28.
Molyneux SL, Young JM, Florkowski CM, Lever M, George PM. Coenzyme Q10: is there a clinical role and a case for measurement? Clin Biochem Rev 2008;29:71–82.
Ozaki A, Muromachi A, Sumi M, Sakai Y, Morishita K, Okamoto T. Emulsification of coenzyme Q10 using gum arabic increases bioavailability in rats and human and improves foodprocessing suitability. J Nutr Sci Vitaminol (Tokyo). 2010;56(1):41-7.
Pepe S, Marasco SF, Haas SJ, Sheeran FL, Krum H, Rosenfeldt FL. Coenzyme Q10 in cardiovascular disease. Mitochondrion. 2007;7 Suppl:S154-67. Epub 2007.
Pravst I, Zmitek K, Zmitek J. Coenzyme Q10 contents in foods and fortification strategies. Crit Rev Food Sci Nutr. 2010;50(4):269-80.
Rosenfeld J, Rodicio JL, Tuomilehto J, Zanchetti A. Randomized double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997;350:757-764.
Rosenfeldt FL, Haas SJ, Krum H, Hadj A, Ng K, Leong JY, Watts GF. Coenzyme Q10 in the treatment of hypertension: a meta-analysis of the clinical trials. J Hum Hypertens. 2007;21(4):297-306. Epub 2007.
Sarter B. Coenzyme Q10 and cardiovascular disease: a review. J Cardiovasc Nurs. 2002;16(4):9-20.
Shah SA, Sander S, Cios D, Lipeika J, Kluger J, White CM. Electrocardiographic and hemodynamic effects of coenzyme Q10 in healthy individuals: a double-blind, randomized controlled trial. Ann Pharmacother. 2007;41(3):420-5. Epub 2007.
SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertention. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991;265:3255-3264.
Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, Bulpitt CJ, de Leeuw PW, Dollery CT, Fletcher AE, Forette F, Leonetti G, Nachev C, O’Brien ET,
Soukoulis V, Dihu JB, Sole M, Anker SD, Cleland J, Fonarow GC, Metra M, Pasini E, Strzelczyk T, Taegtmeyer H, Gheorghiade M. Micronutrient deficiencies an unmet need in heart failure. J Am Coll Cardiol. 2009;54(18):1660-73.
Statistics Canada. Leading causes of death. http://www.statcan.gc.ca/daily-quotidien/101130/ dq101130b-eng.htm. Accessed December 2010.
Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, et al. Primary prevention of hypertension: clinical and public health advisory from the National High Blood Pressure Education Program. JAMA 2002;288(15):1882-8.
Wyman M, Leonard M, Morledge T. Coenzyme Q10: a therapy for hypertension and statininduced myalgia? Cleve Clin J Med. 2010;77(7):435-42.
Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364(9438):937-52.