Bacopa monnieri
Application for cognitive enhancement
Our population demographics are changing – Statistics Canada has projected that by 2056 between 25 – 30 % of the Canadian population will be over the age of 65 (up from 13.7% in 2006) (Statistics Canada 2010). This has both social and economic implications – from the stand point of cognitive health and performance; it is plausible that cognitive enhancing agents may become increasingly sought after by the otherwise healthy aging adult (Calabrese 2008). A recent (2004) community survey of 2551 adults between 60-64 years of age conducted in Australia showed that 2.8% of the sample reported using a non-prescription drug for memory aid or cognitive enhancement. Bacopa monnieri (B. monnieri) was amongst the top three agents used (Jorm 2004).
Bacopa monnieri, also commonly known as Water Hyssop or Brahmi in India is a member of the Scrophulariaceae plant family (Calabrese 2008). It has ancient historical use as a brain tonic for anxiety, memory, poor cognition and concentration in traditional Ayurvedic medicine; chronicles of its use date back to as early as 100 A.D. (Calabrese 2008, Chowdhuri 2002, Usha 2008). B. monnieri has many other purported actions including analgesic, anti-inflammatory, antipyretic, sedative and anti-epileptic properties, however its use as a memory enhancing agent has predominated (Nathan 2001, Stough 2001).
The following review examines available clinical evidence for the use of B. monnieri for cognitive enhancement, specifically for its use in memory augmentation.
Mechanism of action:
The exact mechanism of B. monnieri on psychoneurological parameters remains unknown (Stough 2001). In vitro as well as in vivo animal studies indicate that the effect of B. monnieri on memory appears to be multifocal and suggests that it includes modulation of the cholinergic system, altered antioxidant status as well as anti stress activity by modulation of HSP70, P450 and superoxide dismutase within the brain (Bhattacharya 2000, Roodnenrys 2002, Stough 2001). Most recently B. monnieri has demonstrated neuroprotective effects and cognitive enhancement in preclinical Alzheimer’s disease models (Limpeanchob 2008, Uabundit 2010).
The cognitive enhancing effects of B. monnieri extracts are attributed to the saponins within the plant, specifically bacosides A and B (Joshua- Allan 2008). Research suggests that the bacosides are the component of the plant that are involved in the reversal of amnesic effects of neurotoxins, scopolamine, phenytoin, electroschock, immobilization stress and diazepam as seen in animal studies (Calabrese 2008, Prabhakar 2008).
A rat study showed that B monnieri extract produced a dose-dependent effect on antioxidants superoxide dismutase, catalase and glutathione peroxidase activity in the frontal cortex, striatum and hippocampus of the brain (Bhattacharya 2000). It has been hypothesized that this effect may in part result in enhancement of the cognitive functions associated with those parts of the brain: memory, executive decision making and information processing (Bhattacharya 2000). It should be noted that the standardization of bacosides in this rat study was to bacoside A at 82% +/- 0.5 % which is significantly higher than that used in human trials (bacoside standardization of 40-55%).
Clinical Evidence:
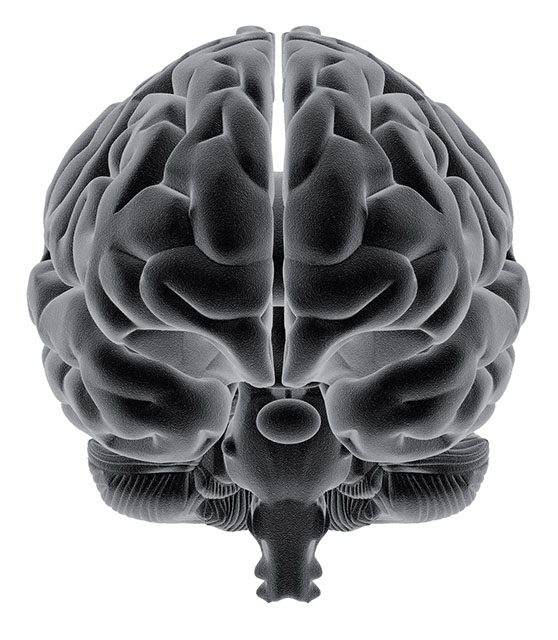
Bacopa monnieri has been the subject of eight double blind randomized placebo controlled trials (n=498) with the primary objective including assessing the effect of B. monnieri on memory. All but two of the trials were conducted on healthy adults ranging from 18 – 65 years of age and lasting up to 16 weeks in duration. Several of the trials also assessed for safety, tolerability as well as anxiety and depression.
In all of the trials, assessments of cognitive enhancement was conducted through a battery of neuropsychological testing including the Rey Auditory Verbal Learning Test (AVLT), which objectively measures audioverbal and visual memory, and the Wechsler Adult Intelligence Scale, but the types and number of assessments employed varied significantly between studies (Morgan 2010).
Raghav (2006) (n=40) and Morgan (2010) (n=98) specifically examined the effect of B. monnieri on adults (> 55 yoa) with age related memory impairment in the absence of diagnosed dementia. Both trials showed statistically significant improvements in the trial groups. Morgan (2010) assessed for audio-verbal and visual memory performance using the AVLT, Rey-Osterrith Complex Figure Test (CFT) and Reitan Trail Making Test (TMT). Subjective memory performance was also assessed. The team found that B. monnieri was able to produce statistically significant improvements in memory acquisition and retention as per AVLT assessment. Raghav and colleagues used the Wechsler memory scale and its subsets and reported improvements in logical memory, mental control and paired associated learning (Raghav 2006).
All but one RCT concluded that B. monnieri produced cognitive enhancement in adults with or without adult age related memory impairment. The trials by Calabrese 2008, Stough 2001, Morgan 2010 and Roodenrys 2002 all used similar dosing of B. monnieri; 300 mg qd for a 12 week duration. All four trials concluded that B. monnieri enhanced cognition in adults; however the specific effects reported on cognition differed between trials (See Table 1).
The study by Stough and colleagues showed improved information processing, learning rate and memory consolidation (2001). Roodenrys 2002 which employed a slight variation in dosing depending on the subject weight (300 mg qd for subjects < 90kg and 405 mg qd for subjects >90 kg) reported improvements in retention of new information; however no improvements were noted in attention, verbal and visual short term memory, or retrieval of pre-existing knowledge or anxiety, which had previously been reported to be improved in the studies conducted by Calabrese 2008 and Stough 2002 (Roodenrys 2002). Morgan 2010 also reported significant improvements in memory acquisition whereas Roodenrys 2002 suggests it is the rate of forgetting that was decreased by B. monnieri (Morgan 2010).
From this is becomes apparent that there is a need for further clinical trials which use the same neuropsychological assessments in order to ascertain the specific effect B. monnieri has on memory – whether it increases fact retention or decreases the rate of forgetting.
The trial that did not show any statistically significant effect of B. monnieri on neuropsychological parameters was investigating the acute (2 hours) effects of the herb on cognitive parameters (Nathan 2001).
Supplementation with B. monnieri may have cognitive enhancing effects even after its intake is discontinued (Rhagav 2006). In one double blind RCT the trial groups were randomized to either B. monnieri or placebo for 12 weeks, after this period the B. monnieri group was also given placebo for a subsequent four weeks; where as the placebo group consumed placebo for the entire 16 week trial (Raghav 2006). Neuropsychological assessments were significantly improved in the B. monnieri group at the end of the trial and no decrease in memory performance scores were noted between week 12 and week 16 time points (Raghav 2006). This study demonstrates that outcomes delivered through B monnieri supplementation persist for at least four weeks following discontinuation of the intervention.
Safety & Dosing:
Dosing varied between the trials (from 250 mg qd to 750 mg qd), with the majority prescribing 300 mg qd of standardized B. monnieri extract either in one single dose or in divided doses. The extracts were standardized to bacoside content, which varied between 40 – 55% (Calabrese 2008, Morgan 2010, Nathan 2001, Raghav 2006, Stough 2001). Two studies used 750 mg qd of which 100 mg was a whole plant extract and the remaining 650 mg was powdered plant (Mandal, Nangelil). In Roodenrys (2002) dosing was done by weight in which persons under 90kg where given 300 mg and those over 90kg were given 450 mg of B. monnieri, which was equated to 6 and 9g of dried B. monnieri rhizome, respectively.
A 2007 toxicological in vivo study in Sprague-Dawley rats reported a median oral lethal dose of 2400mg/kg of B. monnieri extract (Joshua- Allan 2007). Repeated daily oral dosing for 14 days was well tolerated in rats at up to 500mg/kg; furthermore, a sub-chronic study for 90 days with doses up to 500 mg/kg did not result in toxicities in these animals (Joshua- Allan2007).
A phase I safety study on a B. monniere extract has been conducted in 23 healthy human subjects who were given 300 mg of the extract with a dose escalation up to 450 mg qd for a total of 30 days (Morgan 2010, Pravina 2007). Three of the 23 subjects reported gastrointestinal adverse events; the trial concluded that B. monnieri was safe and did not produce either clinical nor laboratory evidence indicating that it was unsafe at 300 mg qd (Pravina 2007).
B. monnieri has a long historical use which speaks to its safety profile; that being said, a small number of adverse events were noted in four of the studies, they included gastrointestinal effects (increased stool frequency, abdominal cramping and nausea) (Calabrese 2008, Morgan, 2010, Pravina 2007, Roodnenrys 2002) fatigue and dry mouth (Stough 2001). It has been hypothesized that the gastrointestinal adverse events may be due to the cholinergic effects of B. monnieri (Morgan 2010).
Conclusion:
At present, clinical as well as laboratory research has shown that B. monnieri appears to have cognitive enhancing effects; however the specific mechanisms of action are not well understood and from the clinical trials completed there appears to be a discrepancy as to how it may impact memory specifically.
There is a large number of limitations to the current research including: small trial sample size, large variation in assessment methods between trials, lack of long term data, inconsistency in dosing and standardization of B. monnieri extract. Thus far the research is encouraging and combined with the historical use of B. monnieri as a cognitive enhancement agent in Ayurvedic medicine, this should provide a basis for further research for this promising agent.
References:
Bhattacharya S.K., Bhattacharya A., Kumar A., Ghosal S. Antioxidant activity of Bacopa monniera in rat frontal cortex striatum and hippocampus. Phytother Res. 2000; 14(3): 174 – 179.
Calabrese C., Gregory W., Leo M., Kraemer D., Bone K., Oken B. Effect of standardized Bacopa monnieri extract on cognitive performance, anxiety, depression in the elderly: randomized, double-blind, placebo-controlled trial. The Journal of Alternative and Complementary Medicine. 2008; 14(6): 707-713.
Chowdhuri DK., Parmar D., Kakkar P., Shukla R., Seth P.K., Srimal R.C. Antistress effecs of Bacosides of Bacopa monnieri: Modulation of Hsp70 expression, superoxide dismutase and cytochrome p450 activity in rat brain. Phytother. Res. 2002; 16: 639-645.
Jorm AF, Rodgers B, Christensen H. Use of medications to enhance memory in a large community sample of 60-64 year olds. Int Psychogeriatr. 2004;16:209–217.
Joshua- Allan J., Damodaran A., Deshmukh N.S., Goudar K.S., Amit A., Safety evaluation of a standardized phytochemical composition extract from Bacopa monnieri in Sprague-Dawley rats. Food and Chemical Toxicology. 2007; 45: 1928-1937.
Limpeanchob N., Jaipan S., Rattanakaruna S., Phrompittayarat W., Inkaninan K., Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death cortical culture. Journal of Ethnopharmacology. 2008; 120: 112 – 117
Mandal A. A clinical study to evaluate the efficacy and safety of Bacopa caplets in memory and learning ability: A double blind placebo controlled study. The Himalayan Drug Company. [Received via e-mail on 29 January 2010]. Unpublished.
Morgan A., Stevens J. Does Bacopa monnieri improve memory performance in older persons? Results of a randomized, placebo controlled, double blind trial. The Journal of Alternative and Complementary Medicine. 2010; 16 (7): 753-759
Nangelil V. Effect of Bacopa caplet on human memory: a double blind placebo controlled study. The Hialayan Drug Company. [ Received via e-mail on 29 January 2010]. Unpublished.
Nathan P.J., Clarke J., Lloyde J., Hutchison C.W., Downey L., Stough C. The acute effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy normal subjects. Hum Psychopharmacol. 2001; 16: 345 – 351.
Prabhakar S., Saraf M.K., Pandhi P., Anand A., Bacopa monniera exerts antiamnesic effec on diazepam induced anterograde amnesia in mice. Psychopharmacology. 2008; 200: 27-37.
Pravina K., Ravindra K.R., Goudar D.R., Vind A.J., Joshua P., Wasim K., Venkatehwarlu V.S., Saxena A.A. Safety evaluation of BacoMind in healthy volunteers: A phase I study. Phytomedicine. 2007; 14:301-308.
Raghav S., Singh H., Dalai P.K., Srivastava J.S., Asthana O.P. Randomized controlled trial of standardized Bacopa monniera extract in age- associated memory impairment. Indian J Psychiatry. 2006; 48(4): 238-242
Roodenrys S., Booth D., Bulzomi S., Phipps A., Micallef C., Smoker J. Chronic effects of Brahmi (Bacopa monnieri) on human memory. Neuropsychopharmacology. 2002; 27: 279 – 281.
Statistics Canada – Demographic Change, 2010 [ Accessed online 20 December 2010 at [http://www.statscan.gc.ca/pub/82-229-x/2009001/demo/int1-eng.htm]
Stough C., Lloyd J., Clarke J., Downey L.A., Hutchison C.W., Rodgers T., Nathan P.J. The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacology. 2001; 156:481-484.
Uabundit N., Wattanathorn J., Supaporn M., Kornkanok I. Cognitive enhancement and neuroprotective effects of Bacopa monnieri in Alzheimer’s disease model. Journal of Ethnopharmacology. 2010; 127: 26-31.
Usha P.D., Wasim P., Joshua J.A., Geetharani P., Murali B., Mayachari A.S., Venkateshwarlu K., Saxena V.S., Deepak M., Amit A. BacoMind: A cognitive enhancer in children requiring individual education programme. Journal of Pharmacology and Toxicology. 2008; 3(4): 302-310.