Polycystic ovary syndrome
Clinical considerations and therapeutic options
Polycystic ovary syndrome (PCOS) is a complex condition of metabolic and endocrine dysfunction. Estimates of its prevalence in women range from 6-18% worldwide (Azziz 2009, Kumarapeli 2008, March 2010), making it one of the most common human disorders (Goodarzi 2011).
The central features of PCOS include clinical hyperandrogenism or biochemical hyperandrogenemia, oligoanovulation and polycystic ovaries (Goodarzi 2011). Three diagnostic schemes appearing in the current literature identify a wide range of PCOS phenotypes involving diverse combinations of hyperandrogenism, hyperandrogenemia and ovarian dysfunction (March 2010). Diagnostic methods and criteria have evolved considerably since PCOS was first described in 1935 by Stein and Leventhal (Azziz 2009), but agreement has yet to be reached as to which symptoms truly define this syndrome.
Clinical presentation
Hyperandrogenism can appear clinically as hirsutism, acne, and androgenic alopecia. Of these three symptoms, hirsutism appears to be most predictive of PCOS, with 75-80% of hirsute women fitting diagnostic criteria for this syndrome (Azziz 2009). Ethnic variation does exist, and fewer East Asians with PCOS present with unwanted hair growth than white or black women (Goodarzi 2011). Clinical symptoms of hyperandrogenism may be supported by laboratory findings (hyperandrogenemia) which may include elevated testosterone and dehydroepiandrosterone sulfate (DHEAS). However, up to 40% of women with PCOS are estimated to have androgen levels within the normal range (Azziz 2009).
Menstrual dysfunction in the form of oligomenorrhea or amenorrhea occurs in 70-80% of women with PCOS (Teede 2010). Dysfunction may not be easily appreciated in women with regularly-occurring but anovulatory periods, and may only be revealed through comprehensive hormonal assessment.
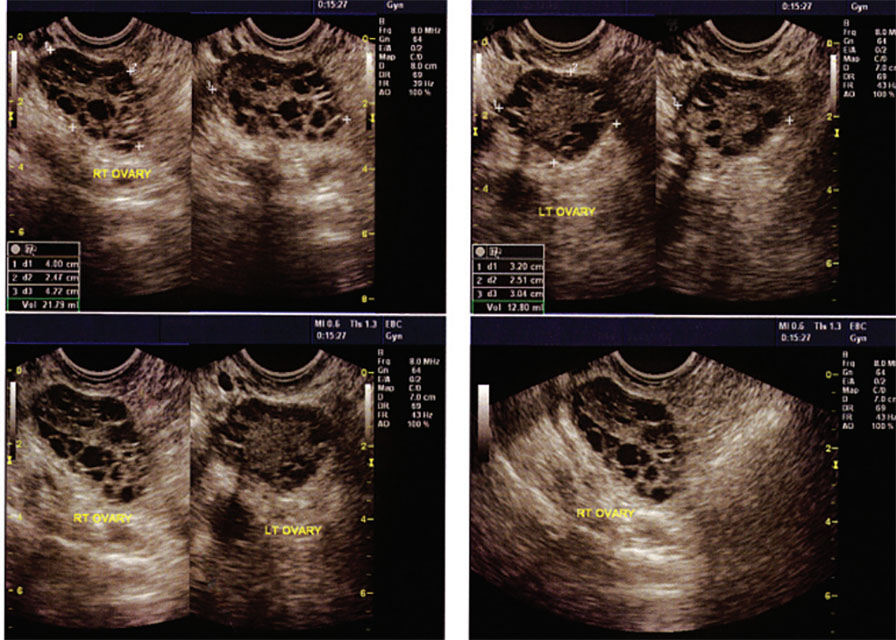
Although polycystic ovaries are present in an estimated 80% of women with PCOS, their identification is not required for diagnosis (March 2010). As asymptomatic polycystic ovaries may be found in up to 30% of the population (Azziz 2009), changes in ovarian morphology, while common, are not an essential feature of PCOS, despite its name.
Diagnosis of exclusion
Alternative pathologies must be excluded before a diagnosis of PCOS may be made. Disturbances in thyroid function can cause changes to the menstrual cycle (Krassas 2000) while hyperprolactinemia is associated with infertility and amenorrhea (Patel 2007). Non-classic congenital adrenal hyperplasia may not appear until the reproductive years, and is associated with hyperandrogenism (Young 2010), while hirsutism may be a sign of Cushing’s syndrome (Shibli-Rahhal 2006). Clinicians must also be attuned to the possibility that these conditions may exist concomitantly.
Associated conditions
Other signs are often associated with PCOS and exacerbate the impact of this syndrome, although they are not required for diagnosis. The most important of these is insulin resistance, and associated hyperinsulinemia. Excess insulin drives endocrine derangement by stimulating androgen production (Bergh 1993), and decreasing hepatic production of sex hormone binding globulin (SHBG) (Goodarzi 2011). Insulin resistance is less commonly observed in lean women with PCOS (Stovall 2011) and is worsened by smoking (Cupisti 2010).
Obesity exacerbates the clinical features of PCOS by promoting insulin resistance (Teede 2010) and unopposed estrogen production (Azziz 2009). The link between PCOS and obesity is amplified further, as insulin-driven hyperandrogenemia, in turn, contributes to abdominal adiposity (Tolino 2005). While increased BMI only marginally increases the risk of developing PCOS (Yildiz 2008), and while as many as 50% of women with PCOS are not obese (Goodarzi 2011), obesity has a significant, negative impact on quality of life in this population (Jones 2008).
Fertility challenges are natural sequelae to any condition affecting ovarian function. PCOS is responsible for up to 70% of cases of anovulatory infertility (Brassard 2008), although as many as 60% of women with PCOS may be able to conceive (Teede 2010). Obesity independently contributes to decreased fertility in women with PCOS (Marquard 2010), and weight loss may improve the chances of conceiving (Crosignani 2003). During pregnancy, women with PCOS are at increased risk for pre-eclampsia, hypertension and gestational diabetes (Goodarzi 2011).
Associated risks
The long-term health effects of PCOS must also be of concern to the clinician:
• PCOS-associated insulin resistance increases the risk of developing Type 2 diabetes, especially in women who are also obese.
• Dyslipidemia, a common finding in US women with PCOS, increases the risk of cardiovascular disease.
• The metabolic syndrome, which incorporates abnormalities in both insulin and cholesterol metabolism, occurs more frequently in US women with PCOS than those without (Goodarzi 2011).
• Increased cardiovascular risk is a concern, even in lean women with PCOS (Costa 2010).
The significance of these risk factors is highlighted by reports of increased incidence of cardiovascular events and decreased event-free survival in this population (Shaw 2008).
Preliminary data show that rates of endometrial cancer may be slightly higher in women with PCOS (Chittenden 2009), and were significantly associated with high BMI, hirsutism and very irregular periods in an Australian case control study (Fearnley 2010). Investigations continue into associations between PCOS and other gynaecological cancers.
Treatment of PCOS – Lifestyle interventions
A recent systematic review (Moran 2011) found that exercise, either alone or in combination with caloric restriction, addressed the exacerbating conditions associated with PCOS. Participants in the trials reviewed lost weight, decreased abdominal obesity, and saw improvements in insulin resistance. A meta-analysis was not performed, but the results appeared to be consistent throughout the trials. None of the included studies reported on reproductive outcomes, but observed improvements in both clinical and biochemical signs of hyperandrogenism suggest an additional impact upon endocrine parameters.
BMI, insulin resistance and serum hormone levels
Other studies confirm these results. Karimzadeh (2010) compared lifestyle changes (caloric deficit of 500kcal/day, combined strength/aerobic training for 20-60 minutes, 3-5 times per week) to pharmacological therapy (metformin and clomiphene citrate (CC)), and reported significant improvements in waist circumference, insulin, LDL and SHBG with the lifestyle intervention. Another small study (n=20) comparing regular aerobic exercise (30 minutes of exercise at 60-70% of the maximal oxygen consumption (VO2max), 3 times per week) to a high protein (35%), hypocaloric diet (deficit of 800kcal/day) reported improvement in both groups in BMI, insulin resistance and waist-hip ratio (WHR) (Palomba 2008).
Weight loss alone, achieved through strict caloric restriction, has been shown to improve insulin (Stamets 2004), SHBG and testosterone levels (Tolino 2005) in women with PCOS. Additionally, nutritional counselling and exercise therapy (90 minutes of combined aerobic/resistance training, 3 times per week) in the absence of weight loss was shown to benefit waist circumference and fasting insulin levels (Bruner 2006) through improved body composition. A combination of these therapies can reasonably be expected to have an additive and positive effect in PCOS patients.
Reproductive outcomes
Improvements in rates of ovulation and pregnancy may also be anticipated with lifestyle changes. A small, prospective trial of 33 overweight women with PCOS, treated with caloric restriction and exercise recommendations, saw improvements in ovulatory function and morphology, and 30% of participants (10/33) achieved pregnancy (Crosignani 2003). Karimzadeh (2010), found parallel rates of ovulation (55-66%) and pregnancy (12-20%) in all treatment arms (described above). Otta (2010) reports statistically significant amelioration of menstrual cycling with caloric restriction and exercise, both in combination with metformin and with placebo (between-group differences were not reported).
Treatment of PCOS – Inositol
Inositol phosphoglycan (IPG) is an insulin mediator influencing the uptake of glucose in the body (Baillargeon 2010). D-chiro inositol (DCI) is an important component of IPG, and defects in the DCI-IPG system have been linked with insulin resistance (Galazis 2011). In women with PCOS, DCI-IPG is less responsive to insulin challenge than in normal controls, and it is speculated that the uncoupling of DCI-IPG with insulin levels may contribute to insulin resistance in this population (Galazis 2011). Given the potential for hyperinsulinemia to exacerbate PCOS, improving insulin sensitivity is an important therapeutic goal.
Studies have investigated the impact of supplementation with inositol, both as DCI or its precursor, myo-inositol (MYO) (Papaleo 2009), in women with PCOS. Daily dosing in published research ranges from 200mg-1200mg of DCI and 2g-4g of MYO.
Although studies are small and heterogeneous, recent reviews (Fritz 2009, Galazis 2011) have described benefit from inositol therapy. The proposed effects of inositol supplementation are reviewed below.
Inositol and insulin resistance
Inositol does appear to benefit glucose metabolism in women with PCOS, likely through its interaction with IPG. Evidence of increased insulin sensitivity (Costantino 2009), decreased insulin resistance (Genazzani 2008) or improved glucose tolerance (Nestler 1999) has been seen in trials using either form of inositol. One researcher also attributed improvements in BMI to inositol, providing a second mechanism through which insulin resistance may be addressed (Gerli 2003, Gerli 2007).
Benefit to clinical features of PCOS
Clinical hyperandrogenism, evidenced by hirsutism and/or acne, improved with 4g MYO supplementation over a 6 month period (Zacche 2009). Hyperandrogenemia, assessed through serum testosterone and DHEAS, was significantly improved by the administration of both MYO (Costantino 2009, Genazzani 2008, Zacche 2009) and DCI (Iuorno 2002, Nestler 1999).
A series of small randomized, controlled trials (RCT) have found significant benefit of both forms of inositol in restoring ovulatory cycles in comparison to placebo, or to metformin (Costantino 2009, Gerli 2007, Nestler 1999 Raffone 2010). These improved rates of ovulation appear to translate into fewer oocytes of poor quality (Ciotta 2010, Papaleo 2009) and a trend towards increased frequency of pregnancies (Morgante 2011), although this latter outcome has yet to reach statistical significance in recent RCTs (Papaleo 2009, Raffone 2010).
Treatment of PCOS – N-acetylcysteine (NAC)
NAC has been shown to affect glucose metabolism (Ammon 1992) and may function as an insulin-sensitizing agent in women with PCOS, possibly through its influence upon intracellular levels of glutathione (GSH) (Fulghesu 2002). Two small trials of NAC as a monotherapy demonstrated improvement in insulin sensitivity following 4-6 weeks of treatment at a dose of 1.8g per day (Fulghesu 2002, Kilic-Okman 2004), although a third trial did not support these findings (Elnashar 2007). Further studies are needed to identify factors impacting the effect of this therapy.
NAC and ovulation induction
The benefit of NAC as an insulin-sensitizing agent may be most apparent in its use as an adjunct to clomiphene citrate (CC). Insulin-sensitizing agents are used to amplify response in resistant patients (Nestler 2000, Rizk 2005), and NAC has shown some promise in this application. Two recent trials found a significant increase in ovulation rates compared to controls (49.3% vs. 1.3% (Rizk 2005) and 52.1% vs. 17.9% (Badawy 2007) when 1.2g of NAC was administered with 100mg of CC.
Other insulin-sensitizing agents such as metformin have been shown to confer greater benefit in terms of ovulation, pregnancy and miscarriage incidence (Abu Hashim 2010), but NAC may still be a valuable option when these agents cannot be used. NAC may also exert its influence through insulin-independent pathways, having the potential for positive effects on vasculature, immune parameters and cervical mucous (Badawy 2007).
The hormonal effects of NAC may be significant in a PCOS-affected population as this agent has been shown to significantly decrease testosterone levels (Elnashar 2007) and increase SHBG (Fulghesu 2002, Kilic-Okman 2004). Fulghesu reported the greatest benefit to insulin levels and hormonal parameters in subjects that were hyperinsulinemic at baseline, indicating that effect of NAC on hormonal levels may be insulin-mediated.
Conclusion
PCOS, a complex, multifaceted condition, presents with varying degrees of endocrine and metabolic dysfunction and poses significant risk of short- and long-term morbidity. Caloric restriction and increases in aerobic and resistance exercise can powerfully impact BMI, WHR, insulin resistance and reproductive outcomes. Dietand lifestyle-based therapies are considered first-line, especially in overweight and obese patients (Franks 2011).
Pharmacological therapies may be used to prompt ovulation and menstruation, antagonize androgens, improve insulin sensitivity, and/or address cardiovascular risk factors. Recent research supports the use of inositol and NAC, both as monotherapies and as adjunctive agents to pharmacotherapy, with demonstrated benefits to insulin sensitivity and hormonal parameters. The wide range of clinical presentations and patient concerns in PCOS provide the clinician with numerous therapeutic targets requiring a truly comprehensive approach to care.
References
Abu Hashim H, Anwar K, El-Fatah RA. N-acetyl cysteine plus clomiphene citrate versus metformin and clomiphene citrate in treatment of clomiphene-resistant polycystic ovary syndrome: a randomized controlled trial. J Womens Health (Larchmt). 2010 Nov;19(11):2043-8.
Ammon HP, Müller PH, Eggstein M, Wintermantel C, Aigner B, Safayhi H, Stützle M, Renn W. Increase in glucose consumption by acetylcysteine during hyperglycemic clamp. A study with healthy volunteers. Arzneimittelforschung. 1992 May;42(5):642-5. (abstract)
Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF; Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS Society. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009 Feb;91(2):456-88.
Badawy A, State O, Abdelgawad S. N-Acetyl cysteine and clomiphene citrate for induction of ovulation in polycystic ovary syndrome: a cross-over trial. Acta Obstet Gynecol Scand. 2007;86(2):218-22.
Baillargeon JP, Iuorno MJ, Apridonidze T, Nestler JE. Uncoupling between insulin and release of a D-chiro-inositol-containing inositolphosphoglycan mediator of insulin action in obese women With polycystic ovary syndrome. Metab Syndr Relat Disord. 2010 Apr;8(2):127-36.
Bergh C, Carlsson B, Olsson JH, Selleskog U, Hillensjö T. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil Steril. 1993 Feb;59(2):323-31.
Brassard M, AinMelk Y, Baillargeon JP. Basic infertility including polycystic ovary syndrome. Med Clin North Am. 2008 Sep;92(5):1163-92, xi.
Bruner B, Chad K, Chizen D. Effects of exercise and nutritional counseling in women with polycystic ovary syndrome. Appl Physiol Nutr Metab. 2006 Aug;31(4):384-91.
Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online. 2009 Sep;19(3):398-405.
Ciotta L, Stracquadanio M, Pagano I, Carbonaro A, Palumbo M, Gulino F. [Effects of inositol on oocyte quality in patients affected with polycystic ovary syndrome.] Minerva Ginecol. 2010 Dec;62(6):525-531. [Article in Italian]
Costa EC, de Sá JC, Soares EM, Lemos TM, Maranhão TM, Azevedo GD. [Evaluation of cardiovascular risk by the LAP index in non-obese patients with polycystic ovary syndrome]. Arq Bras Endocrinol Metabol. 2010 Oct;54(7):630-5. [Article in Portuguese] (abstract)
Costantino D, Minozzi G, Minozzi E, Guaraldi C. Metabolic and hormonal effects of myo-inositol in women with polycystic ovary syndrome: a double-blind trial. Eur Rev Med Pharmacol Sci. 2009 Mar-Apr;13(2):105-10.
Crosignani PG, Colombo M, Vegetti W, Somigliana E, Gessati A, Ragni G. Overweight and obese anovulatory patients with polycystic ovaries: parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum Reprod. 2003 Sep;18(9):1928-32.
Cupisti S, Häberle L, Dittrich R, Oppelt PG, Reissmann C, Kronawitter D, Beckmann MW, Mueller A. Smoking is associated with increased free testosterone and fasting insulin levels in women with polycystic ovary syndrome, resulting in aggravated insulin resistance. Fertil Steril. 2010 Jul;94(2):673-7.
Elnashar A, Fahmy M, Mansour A, Ibrahim K. N-acetyl cysteine vs. metformin in treatment of clomiphene citrate-resistant polycystic ovary syndrome: a prospective randomized controlled study.Fertil Steril. 2007 Aug;88(2):406-9.
Fearnley EJ, Marquart L, Spurdle AB, Weinstein P, Webb PM; Australian Ovarian Cancer Study Group and Australian National Endometrial Cancer Study Group. Polycystic ovary syndrome increases the risk of endometrial cancer in women aged less than 50 years: an Australian case-control study. Cancer Causes Control. 2010 Dec;21(12):2303-8.
Franks S. When should an insulin sensitizing agent be used in the treatment of polycystic ovary syndrome? Clin Endocrinol (Oxf). 2011 Feb;74(2):148-51.
Fritz H. Polycystic ovary syndrome: role of inositol in PCOS management. Integrated Healthcare Practitioners 2009 Oct: 82-87.
Fulghesu AM, Ciampelli M, Muzj G, Belosi C, Selvaggi L, Ayala GF, Lanzone A. N-acetylcysteine treatment improves insulin sensitivity in women with polycystic ovary syndrome. Fertil Steril. 2002 Jun;77(6):1128-35.
Galazis N, Galazi M, Atiomo W. d-Chiro-inositol and its significance in polycystic ovary syndrome: a systematic review. Gynecol Endocrinol. 2011 Apr;27(4):256-62.
Genazzani AD, Lanzoni C, Ricchieri F, Jasonni VM. Myo-inositol administration positively affects hyperinsulinemia and hormonal parameters in overweight patients with polycystic ovary syndrome. Gynecol Endocrinol. 2008 Mar;24(3):139-44.
Gerli S, Mignosa M, Di Renzo GC. Effects of inositol on ovarian function and metabolic factors in women with PCOS: a randomized double blind placebo-controlled trial. Eur Rev Med Pharmacol Sci. 2003 Nov-Dec;7(6):151-9.
Gerli S, Papaleo E, Ferrari A, Di Renzo GC. Randomized, double blind placebo-controlled trial: effects of myo-inositol on ovarian function and metabolic factors in women with PCOS. Eur Rev Med Pharmacol Sci. 2007 Sep-Oct;11(5):347-54.
Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011 Apr;7(4):219-31. Epub 2011 Jan 25.
Iuorno MJ, Jakubowicz DJ, Baillargeon JP, Dillon P, Gunn RD, Allan G, Nestler JE. Effects of d-chiro-inositol in lean women with the polycystic ovary syndrome. Endocr Pract. 2002 Nov-Dec;8(6):417-23.
Jones GL, Hall JM, Balen AH, Ledger WL. Health-related quality of life measurement in women with polycystic ovary syndrome: a systematic review. Hum Reprod Update. 2008 Jan-Feb;14(1):15-25.
Karimzadeh MA, Javedani M. An assessment of lifestyle modification versus medical treatment with clomiphene citrate, metformin, and clomiphene citrate-metformin in patients with polycystic ovary syndrome. Fertil Steril. 2010 Jun;94(1):216-20.
Kilic-Okman T, Kucuk M. N-acetyl-cysteine treatment for polycystic ovary syndrome. Int J Gynaecol Obstet. 2004 Jun;85(3):296-7.
Kumarapeli V, Seneviratne Rde A, Wijeyaratne CN, Yapa RM, Dodampahala SH. A simple screening approach for assessing community prevalence and phenotype of polycystic ovary syndrome in a semi-urban population in Sri Lanka. Am J Epidemiol. 2008 Aug 1;168(3):321-8.
Krassas GE. Thyroid disease and female reproduction. Fertil Steril. 2000 Dec;74(6):1063-70.
March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ.The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010 Feb;25(2):544-51.
Marquard KL, Stephens SM, Jungheim ES, Ratts VS, Odem RR, Lanzendorf S, Moley KH. Polycystic ovary syndrome and maternal obesity affect oocyte size in in vitro fertilization/ intracytoplasmic sperm injection cycles. Fertil Steril. 2010 Nov 10.
Moran LJ, Hutchison SK, Norman RJ, Teede HJ. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011 Feb 16;2:CD007506.
Morgante G, Orvieto R, Di Sabatino A, Musacchio MC, De Leo V. The role of inositol supplementation in patients with polycystic ovary syndrome, with insulin resistance, undergoing the low-dose gonadotropin ovulation induction regimen. Fertil Steril. 2011 Feb 5.
Nestler JE. Obesity, insulin, sex steroids and ovulation. Int J Obes Relat Metab Disord. 2000 Jun;24 Suppl 2:S71-3.
Nestler JE, Jakubowicz DJ, Reamer P, Gunn RD, Allan G. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. N Engl J Med. 1999 Apr 29;340(17):1314-20.
Otta CF, Wior M, Iraci GS, Kaplan R, Torres D, Gaido MI, Wyse EP.Clinical, metabolic, and endocrine parameters in response to metformin and lifestyle intervention in women with polycystic ovary syndrome: a randomized, double-blind, and placebo control trial. Gynecol Endocrinol. 2010 Mar;26(3):173-8.
Papaleo E, Unfer V, Baillargeon JP, Fusi F, Occhi F, De Santis L. Myo-inositol may improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Fertil Steril. 2009 May;91(5):1750-4.
Patel SS, Bamigboye V. Hyperprolactinaemia. J Obstet Gynaecol. 2007 Jul;27(5):455-9.
Palomba S, Giallauria F, Falbo A, Russo T, Oppedisano R, Tolino A, Colao A, Vigorito C, Zullo F, Orio F. Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: a 24-week pilot study. Hum Reprod. 2008 Mar;23(3):642-50.
Raffone E, Rizzo P, Benedetto V. Insulin sensitiser agents alone and in co-treatment with r-FSH for ovulation induction in PCOS women. Gynecol Endocrinol. 2010 Apr;26(4):275-80.
Rizk AY, Bedaiwy MA, Al-Inany HG. N-acetyl-cysteine is a novel adjuvant to clomiphene citrate in clomiphene citrate-resistant patients with polycystic ovary syndrome. Fertil Steril. 2005 Feb;83(2):367-70.
Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, Kelsey SF, Kip KE, Cooper-Dehoff RM, Johnson BD, Vaccarino V, Reis SE, Bittner V, Hodgson TK, Rogers W, Pepine CJ. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health–National Heart, Lung, and Blood Institute sponsored Women’s Ischemia Syndrome Evaluation. J Clin Endocrinol Metab. 2008 Apr;93(4):1276-84.
Shibli-Rahhal A, Van Beek M, Schlechte JA. Cushing’s syndrome. Clin Dermatol. 2006 Jul-Aug;24(4):260-5.
Stamets K, Taylor DS, Kunselman A, Demers LM, Pelkman CL, Legro RS. A randomized trial of the effects of two types of short-term hypocaloric diets on weight loss in women with polycystic ovary syndrome. Fertil Steril. 2004 Mar;81(3):630-7.
Stovall DW, Bailey AP, Pastore LM. Assessment of insulin resistance and impaired glucose tolerance in lean women with polycystic ovary syndrome. J Womens Health (Larchmt). 2011 Jan;20(1):37-43.
Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010 Jun 30;8:41.
Tolino A, Gambardella V, Caccavale C, D’Ettore A, Giannotti F, D’Antò V, De Falco CL. Evaluation of ovarian functionality after a dietary treatment in obese women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2005 Mar 1;119(1):87-93.
Yildiz BO, Knochenhauer ES, Azziz R. Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2008 Jan;93(1):162-8.
Young J, Tardy V, de la Perrière AB, Bachelot A, Morel Y; French Society of Endocrinology. Detection and management of late-onset 21-hydroxylase deficiency in women with hyperandrogenism. Ann Endocrinol (Paris). 2010 Feb;71(1):14-8. (abstract)
Zacchè MM, Caputo L, Filippis S, Zacchè G, Dindelli M, Ferrari A. Efficacy of myo-inositol in the treatment of cutaneous disorders in young women with polycystic ovary syndrome. Gynecol Endocrinol. 2009 Aug;25(8):508-13.