In February, 2015, Theralase Technologies Inc filed for Health Canada approval via a Class 3 Medical Device Licence application after successfully completed the design of the next generation therapeutic laser, the TLC-2000.
According to Health Canada guidelines, approval of a Class 3 Medical Device Licence is expected within approximately 75 days, which would indicate final approval on or about April 2015.
Food and Drug Administration and Conformite Europeene approval of the TLC-2000 will be submitted in this month and approval is expected on or about June 2015.
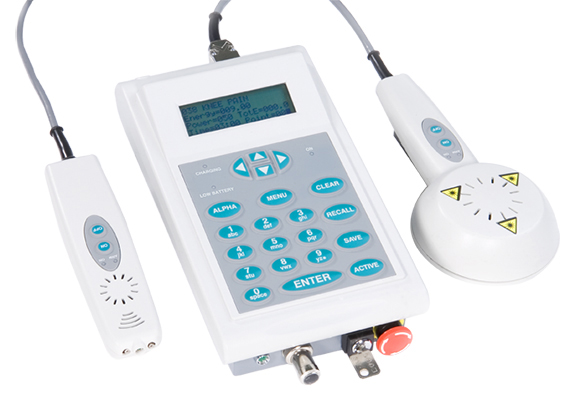
The Theralase TLC-2000 is a next generation patented biofeedback therapeutic laser system that the Company has researched and systematically developed over the last 12 years, commencing with the issue of US patent 6,413,267 on July 2, 2002. Between 2003 to 2009, extensive scientific research of the laser biofeedback technology was completed by analyzing the light propagation and associated attenuation (reduction) through tissue via: computer modeling, phantom technology (materials that optically behave the same as tissue) and finally verification and validation through established animal models.
From 2010 to 2014, Theralase completed the development of the alpha, beta and pre-production level prototypes. The TLC-2000 biofeedback therapeutic laser system possesses patented Cell SensingTM technology, which allows the TLC-2000 the ability to “sense” how deep the damaged tissue is located within the body and then deliver a precise dose of healing light energy to the injured tissue to heal it faster and most effectively than previously attainable. The TLC-2000 automatically adjusts to a patient’s physical characteristics, thus is “patient specific” in the delivery of its energy, adjusting its parameters in relation to each patient treated. The ability to deliver exact doses of light energy to precise depths of target tissue enables the TLC-2000 to provide superior efficacy and accelerated healing for patients, unattainable by any competitive technology. The TLC-2000 is also a learning device that analyzes the clinical efficacy of each treatment delivered, optimizes the clinical protocols accordingly and then relays this new clinical treatment information digitally to each healthcare practitioner, who uses the technology. The healthcare practitioner, regardless of their geographical location, is thus provided with continually optimized clinical treatment protocols to treat their patient’s conditions on a real time basis.
Clinically effective dosages specific to numerous conditions are stored in a Health Insurance Portability and Accountability Act (“HIPAA”) compliant cloud databank and are available real time to all practitioners utilizing the TLC-2000 therapeutic laser system via internet connection to a tablet, which wirelessly connects to the therapeutic laser probe.
By providing healthcare practitioners with a sophisticated medical technology, like the TLC-2000, for delivering laser light energy to tissue, the repeatability and reproducibility of clinical efficacy for each and every patient will be enhanced, an issue that has plagued the therapeutic laser industry for years and prevented this technology from entering mainstream medicine.