GOUT
A Naturopathic Approach
Introduction
Among healthcare providers, gout is often thought of as a relatively benign condition that, while painful acutely, is transient and generally responds well to basic treatments. As a result, gout tends not to receive much attention among healthcare providers, or in the medical literature relative to other arthrides. For those a ected by gout, however, the condition has considerable impact on quality of life. Acute attacks are usually managed e ectively with non-steroidal anti-in ammatory drugs (NSAIDS), but frequent recurrent attacks signal the development of chronic gout (Eggebeen 2007); up to 60% of patients having a gout attack will have a recurrent attack within one year (Eggebeen 2007). Many patients e x p e r i e n c e more frequent a t t a c k s . Gout su erers can sometimes experience frequent attacks despite adhering to a low-purine diet and uric acid lowering therapy. Clinical experience with such a case led this author to review of the biology of gout and available naturopathic treatment options, which are summarized here.
Pathophysiology
Gout is a severely painful in ammatory arthride, most commonly a ecting the large toe (podagra) and more rarely the knee, and is caused by deposits of monosodium urate crystals within the joint space (Smith 2011). Urate crystals may also deposit in soft tissues, causing masses called tophi, and in the kidney, causing nephrolithiasis and urate nephropathy (Eggebeen 2007). Hyperuricemia can be de ned as serum uric acid > or = 7.0 mg/ dl in males and > or = 6.0 mg/dl in females, and a serum level <6.0 mg/dL (355 μmol/L) has been widely accepted as a therapeutic target for urate lowering. is concentration favours dissolution of deposited crystals (Pascual 2007).
The mainstay of uric acid lowering therapy is allopurinol in combination with prescription of a low-purine diet (Pascual 2007). Allopurinol (typically 50-300mg daily) is a purine analog and inhibitor of xanthine oxidase, thereby reducing the conversion of purines to uric acid (Eggebeen 2007, Rx List 2011); the dose of allopurinol should be based on an assessment of kidney function, with up to 300mg per day recommended for those with a creatinine clearance ≥90 mL per minute and less for those with lower kidney clearance (Eggebeen 2007). A low purine diet entails avoidance of meat, and seafood, yeast and yeast containing foods such as beer and alcohol, as well as select purine-rich vegetables including beans, peas, lentils, oatmeal, spinach, asparagus, cauliflower, and mushrooms (Emmerson 1996). Despite these well established treatment strategies, there is a growing recognition that gout is not simply a disorder of purine metabolism, as has traditionally been thought, but a more pervasive condition with considerable overlap with the metabolic syndrome.
Gout and the Metabolic Syndrome
Gout is strongly associated with a number of chronic diseases that also have metabolic components, most notably, metabolic syndrome and its components; hypertension; and cardiovascular disease (Saag 2006). The implications of this are two-fold. First, clinicians treating gout should be alert for such potential co-morbidities, and address them accordingly in order to reduce cardiovascular risk. Secondly, these conditions may share common underlying metabolic etiologies that can potentially be addressed in concert. Saag and Choi propose that obesity and insulin resistance are key factors in contributing to hyperuricemia and gout (Saag 2006).
Large observational studies demonstrate that body mass index (BMI) and insulin resistance have strong correlations with serum urate levels (Rathmann 1998, Saag 2006). In the Coronary Artery Risk Development in Young Adults (CARDIA) study, BMI, fasting insulin, and triglycerides were all significantly higher, and HDL cholesterol lower in subjects with hyperuricemia (p < 0.001 for all). After 10 years of follow up, these associations remained consistent (Rathmann 2007).
In the Verona Young Men Atherosclerosis Risk Factors Study, including 957 18-year old healthy men, Bonora et al found that “Serum uric acid concentration showed positive associations with BMI (r = 0.24; P < 0.0001), WHR (r = 0.19; P < 0.0001)” and other components of metabolic syndrome (1996). BMI was the strongest independent predictor of uric acid. Several additional studies corroborate this relationship across many ethnic groups (Cigolini 1995, Meshkani 2011, Yamada 2011).
Weight reduction reduces serum uric acid (Saag 2006, Dessein 2000, Krzystek-Korpacka 2011, Madero 2011, Yamashita 1986). Obesity can increase production and decrease renal excretion of urate (Yamashita 1986), while a hypocaloric diet decreases serum UA through increased renal excretion (2005). Johnson found that among morbidly obese subjects even a 5% reduction in body weight was associated with global improvements in cardiovascular risk factors as well as serum uric acid, such that uric acid decreased 5.7% with weight loss of between 5%-9.9%, and decreased 16.6% with weight loss of 20% or greater (2011). Again, Birketvedt found that among 53 moderately overweight women, caloric restriction (1200 kcal/d) resulting in between 5-8 kg weight loss led to significant reductions to serum uric acid (2000).
Diet & Lifestyle Recommendations
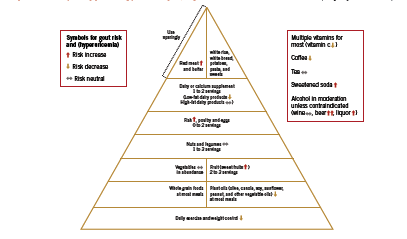
With respect to lifestyle recommendations for gout, Saag and Choi argue that the benefits of a “rigid purine restricted diet” (Saag 2006) must be weighed against the fact that a low-purine diet is often high in refined carbohydrates and saturated fat, potentially aggravating the underlying metabolic factors. Designing a diet that assists patients with weight loss, improves insulin sensitivity and lipid levels, and lowers uric acid is crucial in controlling gout symptoms as well as in managing cardiovascular risk. To this end, Choi has developed a “food pyramid” that incorporates both moderate restriction of purine intake, as well as strategies to improve insulin resistance that are characteristic of the diet used to control metabolic syndrome (2010). An adaptation of this pyramid is shown in Figure 1.
There are some notable inclusions in this dietary plan, namely, moderate amounts of purine rich legumes and vegetables, as well as fish and poultry. Choi argues that plant source purines do not increase risk of gout (2010). In a large observational study of over 47 thousand men, consumption of meat (RR 1.41, 95% CI 1.07-1.86) and seafood (RR 1.51 (1.17-1.95) but not purine rich vegetables and legumes were associated with risk of gout (Choi 2004). Increasing intake of dairy products was associated with reduced risk of gout, RR 0.56 (0.42-0.74). Choi proposes that “it would be difficult to justify a recommendation to avoid all fish intake” (2010) given its benefit on cardiovascular health, however, given the high-purine content of fish, a fish oil supplement (no protein content) seems a better strategy for patients with gout. Soft drinks and sweetened juices are discouraged, however regular consumption of coffee is allowable. Caffeine is a natural xanthine oxidase inhibitor; therefore as long as intake is consistent on a day-to-day basis, coffee may help lower uric acid levels. If intake is variable, however, coffee may in theory worsen gout, since this pattern results in unsteady changes in serum uric acid that may precipitate an attack (2010). Finally, adequate water intake is necessary to ensure uric acid excretion.
Specific Foods and Nutrients
Dairy foods An RCT recently compared the acute effect of four different kinds of milk on serum urate (Dalbeth 2010). Sixteen healthy participants received a single dose containing 80g protein of each of the following: soy milk (control); early season skim milk; late season skim milk, which contains high concentrations of orotic acid, a naturally occurring uricosuric agent; and ultrafiltrated skim milk. Soy increased serum urate approximately 10%, while the other three milks decreased urate approximately 10% (p<0.0001). Authors concluded that the urate-lowering effect of milk was due to its low purine content as well as its ability to increase urinary excretion of uric acid. In addition, certain dairy fractions including glyco-macropeptide and a milk fat extract possess anti-inflammatory properties in experimental models of acute gout (Dalbeth 2011).
Cherries A trial investigating the effect of cherry consumption was conducted in 10 healthy women (Jacob 2003). Subjects consumed two servings (280 g) of sweet cherries after an overnight fast, followed by periodic blood and urine testing. At 5h after cherry consumption, plasma urate decreased to 183 +/-15 micromol/L, compared to 214 +/-13 micro mol/L at baseline (P < 0.05). Urinary excretion of urate increased following cherries, with peak excretion of 350 +/-33 micro mol/mmol creatinine, compared to 202 +/-13 at baseline (P < 0.01).
Black tea/ Coffee In an RCT, three cups of black tea per day resulted in a significant decrease in serum uric acid such that those with baseline levels > 6 mg/dL had a 8.5% decrease (p < 0.05); while those with serum uric acid > 7 mg/dL had a decrease of 9.4% (men) and 7.1% (women) (Bahorun 2010). Coffee has been associated with decreased risk of gout, RR 0.41 (95% CI 0.19-0.88) for six or more cups per day (Choi 2007), as is expected based on its inhibition of xanthine oxidase. Since diuretic agents are known to aggravate gout, it is advisable to recommend a concomitant increase in water intake to patients consuming diuretic beverages in order to support adequate renal clearance.
Eicosapentanoic acid Although no human trials have investigated the effects of fish-derived omega-3 fatty acids on serum uric acid or risk of gout, there is good rationale for recommending EPA to gout patients on the basis of its cardioprotective and anti-inflammatory effects (Choi 2010). Studies in animal models of gout have shown that a combination of EPA and GLA reduced monosodium urate crystal-induced inflammation (Tate 1988).
Vitamin C Vitamin C is a relatively well recognized uricosuric agent, meaning that it promotes urinary excretion of uric acid. A recent meta analysis of 13 RCTs including 556 subjects and a median vitamin C dose of 500 mg/day found that there was a significant reduction in serum uric acid of -0.35 mg/dl (95%CI -0.66 to -0.03, p=0.032); in SI units this was equal to -20.8 μmoles/L (Juraschek 2011). Among trials that were placebo controlled, there were larger reductions in uric acid. Baseline serum uric acid values ranged from 2.9-7.0 mg/dl or 172.5- 416.4 μmoles/L.
Role of Food Sensitivities Although there is no reports in the literature on the association between food sensitivities and uric acid/ gout, the inflammatory state created by an immune reaction to food may aggravate gout-related inflammation and joint pain. For the case mentioned in the introduction, gluten sensitivity seems to have been an important contributing factor to the patient’s gout symptoms. These resolved dramatically on a gluten free diet combined with a selection of the other recommendations discussed here, and notably, subsequent re-exposure to gluten – while continuing other interventions, exercise, and dietary guidelines – led to a clear aggravation of the patient’s gout.
A Note on Drugs Because patients susceptible to gout generally have other cardiovascular risk factors such as hypertension and dyslipidemia, they are often given diruetics and/ or low-dose aspirin; however these medications may potentially precipitate gout. In a prospective study of 5789 hypertensive patients, those treated with a thiazide or loop diuretic had significantly increased risk of developing gout; HR for use of any diuretic 1.48 (95% CI 1.11-1.98), thiazide diuretic use HR 1.44 (95% CI 1.00- 2.10), and loop diuretic HR 2.31 (95% CI 1.36-3.91) (Demarco 2011). This relationship between diuretics and gout is well recognized (Saag 2006). Aspirin has been found to have a bimodal effect on excretion of uric acid: while at high doses (>3g/d) it promotes uric acid excretion (uricosuric action), at low doses (1-2g/d) it has been shown to increase retention of uric acid (Saag 2006). In another study, 75mg aspirin per day led to a 15% decrease in the rate of uric acid excretion (p=0.045) and increase in serum uric acid (p=0.009) (Caspi 2000, Saag 2006).
Conclusion Gout strongly impacts quality of life in affected patients. Because hyperuricemia is associated with the metabolic syndrome and cardiovascular risk factors, dietary strategies for gout need to be balanced in order to reduce purine intake but also reduce risk of cardiovascular disease. Weight loss is key in lowering uric acid levels. Therapeutic foods and nutritional supplements for gout include low fat dairy, black tea/ coffee, cherries, EPA, and vitamin C. The potential role of food sensitivities and medications should also be considered.
References
Bahorun T, Luximon-Ramma A, Gunness TK, Sookar D, Bhoyroo S, Jugessur R, Reebye D, Googoolye K, Crozier A, Aruoma OI. Black tea reduces uric acid and C-reactive protein levels in humans susceptible to cardiovascular diseases. Toxicology. 2010 Nov 28;278(1):68-74.
Birketvedt GS, Aaseth J, Florholmen JR, Ryttig K. Long-term effect of fibre supplement and reduced energy intake on body weight and blood lipids in overweight subjects. Acta Medica (Hradec Kralove). 2000;43(4):129-32.
Caspi D, Lubart E, Graff E, Habot B, Yaron M, Segal R. The effect of mini-dose aspirin on renal function and uric acid handling in elderly patients. Arthritis Rheum. 2000 Jan;43(1):103-8.
Cigolini M, Targher G, Tonoli M, Manara F, Muggeo M, De Sandre G. Hyperuricaemia: relationships to body fat distribution and other components of the insulin resistance syndrome in 38-year-old healthy men and women. Int J Obes Relat Metab Disord. 1995 Feb;19(2):92-6.
Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol. 2010 Mar;22(2):165-72.
Choi HK, Willett W, Curhan G. Coffee consumption and risk of incident gout in men: a prospective study. Arthritis Rheum. 2007 Jun;56(6):2049-55.
Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005 Apr 11;165(7):742-8.
Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004 Mar 11;350(11):1093-103.
Dalbeth N, Wong S, Gamble GD, Horne A, Mason B, Pool B, Fairbanks L, McQueen FM, Cornish J, Reid IR, Palmano K. Acute effect of milk on serum urate concentrations: a randomised controlled crossover trial. Ann Rheum Dis. 2010 Sep;69(9):1677-82.
Dalbeth N, Palmano K. Effects of dairy intake on hyperuricemia and gout. Curr Rheumatol Rep. 2011 Apr;13(2):132-7.
Demarco MA, Maynard JW, Baer AN, Gelber AC, Young JH, Alonso A, Coresh J. Diuretic use, increased serum urate and the risk of incident gout in a populationbased study of hypertensive adults: The atherosclerosis risk in the communities cohort. Arthritis Rheum. 2011 Sep 27. doi: 10.1002/art.33315.
Emmerson BT. The management of gout. N Engl J Med. 1996 Feb 15;334(7):445-51.
Gibson T. Hyperuricemia, gout and the kidney. Curr Opin Rheumatol. 2011 Dec 12. [Epub ahead of print]
Inokuchi T, Tsutsumi Z, Takahashi S, Ka T, Moriwaki Y, Yamamoto T. Increased frequency of metabolic syndrome and its individual metabolic abnormalities in Japanese patients with primary gout. J Clin Rheumatol. 2010 Apr;16(3):109-12.
Jacob RA, Spinozzi GM, Simon VA, Kelley DS, Prior RL, Hess-Pierce B, Kader AA. Consumption of cherries lowers plasma urate in healthy women. J Nutr. 2003 Jun;133(6):1826-9.
Juraschek SP, Miller ER 3rd, Gelber AC. Effect of oral vitamin C supplementation on serum uric acid: a meta-analysis of randomized controlled trials. Arthritis Care Res (Hoboken). 2011 Sep;63(9):1295-306.
Johnson WD, Brashear MM, Gupta AK, Rood JC, Ryan DH. Incremental weight loss improves cardiometabolic risk in extremely obese adults. Am J Med. 2011 Oct;124(10):931-8.
Krzystek-Korpacka M, Patryn E, Kustrzeba-Wojcicka I, Chrzanowska J, Gamian A, Noczynska A. The effect of a one-year weight reduction program on serum uric acid in overweight/obese children and adolescents. Clin Chem Lab Med. 2011 May;49(5):915-21.
Madero M, Arriaga JC, Jalal D, Rivard C, McFann K, Pérez-Méndez O, Vázquez A, Ruiz A, Lanaspa MA, Jimenez CR, Johnson RJ, Lozada LG. The effect of two energyrestricted diets, a low-fructose diet versus a moderate natural fructose diet, on weight loss and metabolic syndrome parameters: a randomized controlled trial. Metabolism. 2011 Nov;60(11):1551-9.
Meshkani R, Zargari M, Larijani B. The relationship between uric acid and metabolic syndrome in normal glucose tolerance and normal fasting glucose subjects. Acta Diabetol. 2011 Mar;48(1):79-88.
Pascual E, Sivera F. Therapeutic advances in gout. Curr Opin Rheumatol. 2007 Mar;19(2):122-7.
Rathmann W, Funkhouser E, Dyer AR, Roseman JM. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. Ann Epidemiol. 1998 May;8(4):250-61.
Rx List Online. Zyloprim (Allopurinol). Updated 2011. http://www.rxlist.com/ zyloprim-drug.htm Accessed December 22 2011.
Saag KG, Choi H. Epidemiology, risk factors, and lifestyle modifications for gout. Arthritis Res Ther. 2006;8 Suppl 1:S2.
Smith HS, Bracken D, Smith JM. Gout: current insights and future perspectives. J Pain. 2011 Nov;12(11):1113-29.
Tate GA, Mandell BF, Karmali RA, Laposata M, Baker DG, Schumacher HR Jr, Zurier RB. Suppression of monosodium urate crystal-induced acute inflammation by diets enriched with gamma-linolenic acid and eicosapentaenoic acid. Arthritis Rheum. 1988 Dec;31(12):1543-51.
Yamada T, Fukatsu M, Suzuki S, Wada T, Joh T. Elevated serum uric acid predicts impaired fasting glucose and type 2 diabetes only among Japanese women undergoing health checkups. Diabetes Metab. 2011 Jun;37(3):252-8.