Female Fertility
Oxidant stress and a potential role for antioxidant therapy
Infertility affects up to 15% of Canadian couples and may be attributed to a number of diverse factors. Recent evidence suggests that while oxidative processes play an essential role in human reproduction, a state of oxidative stress may contribute significantly to the inability to conceive.Oxidative stress has been implicated in endometriosis, recurrent pregnancy loss and poor embryo quality. Human studies into the effect of antioxidants upon reproductive outcomes have shown some promising results. Interventions including vitamin A, vitamin E, n-acetyl cysteine, and melatonin may quench oxidative stress, while observational data suggests a possible role for CoQ10. Antioxidants may offer novel therapeutic options in the management of female infertility.
Infertility is defined as a “failure to achieve a successful pregnancy after 12 months or more of regular unprotected intercourse” (PCASRM 2008). Recent surveys suggest that between 11.5% and 15.7% of Canadian couples attempting to get pregnant are dealing with an inability to conceive, with prevalence rates increasing with advancing maternal age (Bushnik 2012). It is estimated that approximately 30% of infertility causes may be attributed to male factors and 40% to female factors. In the remainder of cases, a combination of influences or an undetermined cause are deemed to be responsible (AHRC 2010).
Through diagnostic imaging and laboratory assessment, many causes of female infertility may be identified and treated. Common causes include disturbances to ovulation caused by polycystic ovarian syndrome (PCOS) and its accompanying insulin resistance and androgen dominance, and disruptions to the physical structure of the reproductive tract as a result of fibroids, endometriosis and pelvic inflammatory disease. Factors external to the reproductive tract such as age, genetics, smoking status and toxin exposure history can also influence the viability of reproductive cells and the success of attempts at pregnancy.
Endocrine functioning, involving the complex interplay of reproductive hormones (estrogen, progesterone, luteinizing hormone and follicle stimulating hormone), thyroid hormones (thyroid stimulating hormone, T3, T4, reverse T3), prolactin, melatonin, insulin and cortisol, has an equally important role in determining the success or failure of any attempts at conception. This component presents perhaps the greatest challenge to those working to support conception. Factors such as body composition, dietary choices, exercise and exposure to stress may have significant impacts on the functioning of this elegant, dynamic system.
In spite of our ability to image, measure and quantify so many aspects of the reproductive tract, a clear cause of infertility cannot be identified in a number of cases (Ledger 2009). These unexplained cases may be attributed in the future to other pathological processes that are the subject of current research including immune functioning (Siam 2011), genetic enzymatic variants (Eloualid 2012) and signaling peptides (Sadeu 2012). A role of oxidative stress in infertility has also been proposed by numerous authors (Agarwal 2005, Ruder 2009, Visioli 2011) and forms the focus of this present analysis.
Free radicals and oxidative stress
Free radicals, comprising two main classes of reactive oxygen species (ROS) and reactive nitrogen species (RNS) are unstable compounds that are produced through many physiological processes. While they are essential to some functions of the body such as infection control (Valko 2007), free radicals have the potential to cause significant tissue damage and disease. For this reason, mechanisms within the body work to stabilize free radicals and to neutralize the damage they may cause. Antioxidant compounds and enzymes accomplish this function on an ongoing basis (Agarwal 2005). When the burden of reactive species overwhelms the compensatory mechanisms of the body, oxidative stress occurs, causing damage to cellular structures and DNA (Valko 2007).
Oxidative processes in reproduction
As in the rest of the body, oxidative processes are integral to the proper functioning of the reproductive system. Key functions such as follicle and oocyte follicular development, embryonic development and implantation (Agarwal 2005, Wiener-Megnazi 2011) involve ROS. However, as oxidative stress has also been implicated as a causative factor in age-related fertility decline (Keefe 2009), it is clear that oxidative processes are not wholly supportive of reproductive processes.
Oxidative stress and endometriosis
Oxidative stress has been put forth as a contributing factor in women with endometriosis, a significant cause of female infertility (Augoulea 2009). Women with this presentation have been found to have a lower antioxidant capacity, as evidenced by decreased levels of plasma superoxide dismustase in one recent investigation (Prieto 2012). Oxidative processes, originating in the peritoneum, are thought to contribute to the development of endometriosis (Gupta 2005, Lousse 2012) and affect not only the structure of the reproductive tract, but oocyte quality in these women (Saito 2002). It has also been suggested that more advanced cases are associated with more evidence of systemic oxidative stress (Andrade 2010).
The administration of antioxidant therapies may provide a novel strategy for the management of this reproductive concern. One recent trial demonstrated that levels of malondialdehyde (MDA), a marker of oxidative stress, could be significantly attenuated by low doses of vitamins A and E (343mg and 84mg respectively) over a six-month period (Mier-Cabrera 2008). At the end of the study, pregnancy rates were slightly higher in the treatment group but results did not reach significance. Future trials with higher doses of targeted antioxidant compounds may hold promise in the treatment of this common cause of female infertility.
Recurrent pregnancy loss (RPL)
Recurrent pregnancy loss, the spontaneous termination of three or more pregnancies under 20 weeks gestation (Gupta 2007), may also prove to have an association with oxidative mechanisms. One study of 45 women that had experienced recurrent pregnancy loss found significantly decreased total antioxidant capacity (TAC) and increased total oxidative status (TOS) among these women compared to healthy pregnant controls (Toy 2010). This evidence is supported by an earlier study identifying low activity of the paraoxonase-1 enzymatic system that prevents lipid oxidation compared to healthy controls (p<0.01). High levels of lipid hydroperoxide in these same women demonstrated increased levels of oxidative stress in these individuals (p<0.01). It should be noted here that other studies have identified oxidative stress as a result rather than a cause of RPL (Baban 2010).
N-acetyl cysteine (NAC) is a mucolytic compound that has been discussed previously in reference to its application in polycystic ovarian syndrome (PCOS) (Flower 2011), where it appears to improve insulin sensitivity and reduce resistance to clomiphene therapy (Abu Hashim 2010). A recent prospective study (Amin 2008) assessed the suitability of NAC in the treatment of RPL, reporting a significant decrease in the risk of pregnancy loss when treatment with a combination of NAC (0.6g) and folic acid (500mcg) was initiated at the time of pregnancy confirmation. In comparison to folic acid therapy alone, the addition of NAC greatly improved the rate of pregnancy maintenance up to 20 weeks (RR 2.9, 95% confidence interval (CI) 1.5-5.6). Perhaps most importantly, the so-called take home baby rate was significantly higher in the NAC-treated group (RR 1.98, 95%CI 1.3-4.0).
Given the established antioxidant activity of NAC (Amin 2008), coupled with the evidence of increased oxidative stress in women suffering RPL, it is reasonable to postulate that this intervention improved pregnancy outcomes by attenuating the predominance of oxidative reactions in the body. It is not yet known whether this effect is achieved through direct action of NAC as a scavenger of free radicals or whether it counters oxidation more indirectly by increasing endogenous glutathione levels (Amin 2008). NAC and other antioxidants may have an important role to play in the management of this devastating condition.
Oxidative factors and in-vitro fertilization (IVF)
IVF, with or without intracytoplasmic sperm injection (ICSI), provides hope for many couples struggling with fertility challenges. A review of the literature pertaining to the oxidative status of women undergoing IVF indicates that this population may be another arena where intervention with antioxidant therapies may be appropriate.
Observational data from a series of prospective trials have examined markers of oxidative activity in women undergoing both IVF and ICSI (Bedaiwy 2010, Bedaiwy 2011, Liu 2010) and their relationship to pregnant cycles. All reviewed trials report similar findings. In their assessment of follicular fluid, Bedaiwy et al. (2011) report significant associations between both lower levels of ROS and higher TAC and pregnant cycles. Non-pregnant patients in the study by Liu et al. had higher MDA measurements (p<0.05) and lower SOD levels (p<0.05), indicating higher amounts of oxidative activity. An earlier study of ROS levels in embryo culture dishes found that lower levels on day three were significantly associated with pregnant cycles (Bedaiwy 2010). A milieu dominated by oxidative processes does not appear to favour conception.
Some researchers have taken this concept of hindrance by oxidation a step further and have declared a cut point for ROS levels in follicular fluid (Jana 2010). After assessing ROS in women with a range of fertility concerns (PCOS, endometriosis, tubal factor infertility), Jana et al. propose that ROS levels over 107cps/400micromol follicular fluid do not favour the growth of viable embryos. One may speculate that these findings could eventually translate into improved clinical tools for predicting IVF success rates.
Melatonin and IVF
Melatonin is a hormone that is naturally secreted by the pineal gland to manage sleep/wake cycles in humans. This compound has also been studied extensively for its antioxidant properties (Tan 2007). In light of the evidence suggesting a detrimental effect of oxidation in IVF cycles, it is not surprising that this antioxidant hormone may promote favourable outcomes for individuals undergoing this therapy.
Three recent randomized controlled trials (RCTs) have evaluated the effect of 3mg of melatonin upon IVF-related parameters (Eryilmaz 2011, Rizzo 2010, Tamura 2008). A fourth trial did not specify dose in the abstract that was available for review (Batioğlu 2012). Trials reported significantly higher numbers of mature oocytes at pickup and higher quality embryos in treated patients (Batioğlu 2012, Rizzo 2010, Eryilmaz 2011). Trends towards higher pregnancy rates were also reported. When comparing current and previous IVF cycles, improved fertilization rates were seen among participants receiving melatonin (Tamura 2008).
The benefit of melatonin to parameters of successful IVF therapy may be attributed at least in part to its action as an antioxidant (Eryilmaz 2011, Rizzo 2010). This theory is supported and elaborated upon by a randomized trial (Taketani 2011) that demonstrates a protective effect of melatonin against reactive oxygen species (ROS) such as H2O2. The production of progesterone by luteinized granulosa cells was inhibited in vitro by H2O2 but this effect was reversed with the addition of melatonin. This effect was demonstrated in vivo by the same authors, through the restoration of deficient progesterone levels in some participants treated with 3mg of melatonin (see Figure 1).
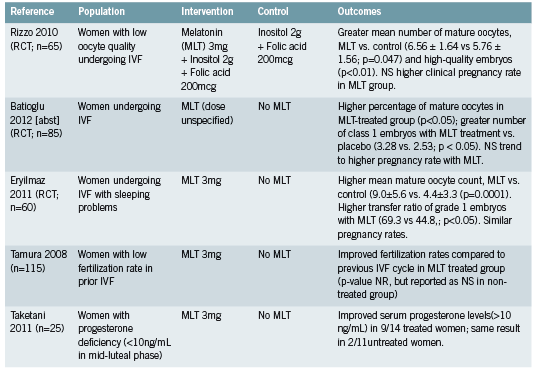
Viewed from this perspective, melatonin may benefit women undergoing IVF, and presumably those trying to conceive naturally as well, through two related mechanisms. First, acting as a free-radical scavenger, melatonin may quench some of the oxidative stress that has been shown to be higher in women with diverse fertility challenges. Secondly, melatonin may reduce the activity of ROS such as H2O2 at the level of the ovary, preventing interference with endogenous progesterone production.
Future directions – CoQ10
While intervention studies have not yet been conducted, coenzyme Q10 (CoQ10) is another antioxidant that may also hold some promise in the treatment of infertility. A recent study investigated CoQ10 levels in the follicular fluid of women undergoing oocyte retrieval. Women with higher levels of CoQ10 had significantly increased numbers of mature oocytes and grade I-II embryos (Turi 2012), suggesting that the presence of this antioxidant compound may also contribute to positive fertility-associated outcomes.
Conclusions
Although oxidative processes are required for numerous essential physiological functions, a state of oxidative stress appears to be associated with conditions that may present barriers to successful conception. Markers of oxidative activity may be higher in women with endometriosis and a history of EPL. While antioxidant therapy has not been fully evaluated for either condition, treatment with 600mg of NAC may help women with EPL to prolong and preserve their pregnancies.
In women undergoing IVF treatments, increased levels of oxidative stress have been observed and may be associated with the success of individual IVF cycles. Melatonin administration at a dose of 3mg per night has been significantly associated with improved oocyte maturity and embryo quality. CoQ10 may also contribute positively to these outcomes but intervention studies are needed to support observational data. While the association between oxidation and female fertility is not entirely understood, this relationship offers some novel therapeutic options to care providers and may further our understanding of infertility that is otherwise unexplained.
References
Abu Hashim H, Anwar K, El-Fatah RA. N-acetyl cysteine plus clomiphene citrate versus metformin and clomiphene citrate in treatment of clomiphene-resistant polycystic ovary syndrome: a randomized controlled trial. J Womens Health (Larchmt). 2010 Nov;19(11):2043-8.
Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction.ReprodBiolEndocrinol. 2005 Jul 14;3:28.
Amin AF, Shaaban OM, Bediawy MA. N-acetyl cysteine for treatment of recurrent unexplained pregnancy loss.Reprod Biomed Online. 2008 Nov;17(5):722-6.
Andrade AZ, Rodrigues JK, Dib LA, Romão GS, Ferriani RA, Jordão Junior AA, Navarro PA.[Serum markers of oxidative stress in infertile women with endometriosis]. [Article in Portuguese] Rev Bras Ginecol Obstet. 2010 Jun;32(6):279-85.
Assisted Human Reproduction Canada (AHRC). Infertility and Assisted Human Reproduction (AHR) [Internet] 2010 Aug 26 [cited 2012 Sep 23]. Available from: http://www.ahrc-pac.gc.ca/v2/patients/infertilityinfertilite- eng.php
Augoulea A, Mastorakos G, Lambrinoudaki I, Christodoulakos G, Creatsas G. The role of the oxidative-stress in the endometriosis-related infertility.GynecolEndocrinol. 2009 Feb;25(2):75-81.
Baban RS. Oxidative stress in recurrent pregnancy loss women. Saudi Med J. 2010 Jul;31(7):759-63.
Batioğlu AS, Sahin U, Gürlek B, Oztürk N, Unsal E. The efficacy of melatonin administration on oocyte quality.GynecolEndocrinol. 2012 Feb;28(2):91-3. [abst]
Bedaiwy MA, Elnashar SA, Goldberg JM, Sharma R, Mascha EJ, Arrigain S, Agarwal A, Falcone T. Effect of follicular fluid oxidative stress parameters on intracytoplasmic sperm injection outcome. GynecolEndocrinol. 2012 Jan;28(1):51-5.
Bedaiwy MA, Mahfouz RZ, Goldberg JM, Sharma R, Falcone T, Abdel Hafez MF, Agarwal A. Relationship of reactive oxygen species levels in day 3 culture media to the outcome of in vitro fertilization/intracytoplasmic sperm injection cycles.FertilSteril. 2010 Nov;94(6):2037-42.
Bushnik T, Cook JL, Yuzpe AA, Tough S, Collins J. Estimating the prevalence of infertility in Canada. Hum Reprod. 2012 Mar;27(3):738- 46.
Eloualid A, Abidi O, Charif M, El Houate B, Benrahma H, Louanjli N, Chadli E, Ajjemami M, Barakat A, Bashamboo A, McElreavey K, Rhaissi H, Rouba H. Association of the MTHFR A1298C variant with unexplained severe male infertility. PLoS One. 2012;7(3):e34111.
Eryilmaz OG, Devran A, Sarikaya E, Aksakal FN, Mollamahmutoğlu L, Cicek N. Melatonin improves the oocyte and the embryo in IVF patients with sleep disturbances, but does not improve the sleeping problems. J Assist Reprod Genet. 2011 Sep;28(9):815-20.
Flower G. Polycystic ovarian syndrome – Clinical considerations and therapeutic options. Integrated Healthcare Practitioners. 2011 April/ May;20:68-72.
Gupta S, Agarwal A, Banerjee J, Alvarez JG. The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: a systematic review. ObstetGynecolSurv. 2007 May;62(5):335-47; quiz 353-4.
Gupta S, Agarwal A, Krajcir N, Alvarez JG. Role of oxidative stress in endometriosis.Reprod Biomed Online. 2006 Jul;13(1):126-34.
Jana SK, K NB, Chattopadhyay R, Chakravarty B, Chaudhury K. Upper control limit of reactive oxygen species in follicular fluid beyond which viable embryo formation is not favorable. ReprodToxicol. 2010 Jul;29(4):447-51.
Keefe DL, Liu L. Telomeres and reproductive aging.ReprodFertil Dev. 2009;21(1):10-4. Ledger WL. Demographics of infertility.Reprod Biomed Online. 2009;18Suppl 2:11-4.
Liu J, Li Y. [Effect of oxidative stress and apoptosis in granulosa cells on the outcome of IVF-ET]. [Article in Chinese] Zhong Nan Da XueXueBao Yi Xue Ban. 2010 Sep;35(9):990-4. [abst]
Lousse JC, Van Langendonckt A, Defrere S, Ramos RG, Colette S, Donnez J. Peritoneal endometriosis is an inflammatory disease. Front Biosci (Elite Ed). 2012 Jan 1;4:23-40.
Mier-Cabrera J, Genera-García M, De la Jara-Díaz J, Perichart- Perera O, Vadillo-Ortega F, Hernández-Guerrero C. Effect of vitamins C and E supplementation on peripheral oxidative stress markers and pregnancy rate in women with endometriosis. Int J Gynaecol Obstet. 2008 Mar;100(3):252-6.
Practice Committee of the American Society for Reproductive Medicine (PCASRM). Definitions of infertility and recurrent pregnancy loss. FertilSteril. 2008 Nov;90(5 Suppl):S60.
Prieto L, Quesada JF, Cambero O, Pacheco A, Pellicer A, Codoceo R, Garcia-Velasco JA. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. FertilSteril. 2012 Jul;98(1):126-30.
Rizzo P, Raffone E, Benedetto V. Effect of the treatment with myoinositol plus folic acid plus melatonin in comparison with a treatment with myo-inositol plus folic acid on oocyte quality and pregnancy outcome in IVF cycles. A prospective, clinical trial.Eur Rev Med Pharmacol Sci. 2010 Jun;14(6):555-61.
Ruder EH, Hartman TJ, Goldman MB.Impact of oxidative stress on female fertility.CurrOpinObstet Gynecol. 2009 Jun;21(3):219-22.
Sadeu JC, DoedéeAM, Neal MS, Hughes EG, Foster WG. Neurotrophins (BDNF and NGF) in follicular fluid of women with different infertility diagnoses.Reprod Biomed Online. 2012 Feb;24(2):174-9.
Saito H, Seino T, Kaneko T, Nakahara K, Toya M, Kurachi H. Endometriosis and oocyte quality. GynecolObstet Invest. 2002;53Suppl 1:46-51.
Siam EM, Hefzy EM.The relationship between antisperm antibodies prevalence and genital chlamydia trachomatis infection in women with unexplained infertility.Afr J Reprod Health. 2011 Sep;15(3):93-101.
Taketani T, Tamura H, Takasaki A, Lee L, Kizuka F, Tamura I, Taniguchi K, Maekawa R, Asada H, Shimamura K, Reiter RJ, Sugino N. Protective role of melatonin in progesterone production by human luteal cells. J Pineal Res. 2011 Sep;51(2):207-13. doi: 10.1111/j.1600- 079X.2011.00878.x. [abst]
Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, Morioka H, Ishikawa H, Reiter RJ, Sugino N. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008 Apr;44(3):280-7. [abst]
Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007 Jan;42(1):28-42.
Toy H, Camuzcuoglu H, Camuzcuoglu A, Celik H, Aksoy N. Decreased serum prolidase activity and increased oxidative stress in early pregnancy loss. GynecolObstet Invest. 2010;69(2):122-7.
Turi A, Giannubilo SR, Brugè F, Principi F, Battistoni S, Santoni F, Tranquilli AL, Littarru G, Tiano L.Coenzyme Q10 content in follicular fluid and its relationship with oocyte fertilization and embryo grading. Arch Gynecol Obstet. 2012 Apr;285(4):1173-6.
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44-84.
Visioli F, Hagen TM. Antioxidants to enhance fertility: role of eNOS and potential benefits. Pharmacol Res. 2011 Nov;64(5):431-7.
Wiener-Megnazi Z, Reznick AZ, Lahav-Baratz S, Shiloh H, Koifman M, Grach B, Arnon T, Avraham L, Auslander R, Dirnfeld M. [Oxidation and female reproduction: the good, the bad and what’s between]. [Article in Hebrew] Harefuah. 2011 Mar;150(3):255-9, 303.









